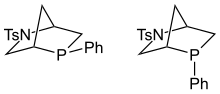
P-Chiral phosphines are organophosphorus compounds of the formula PRR′R″, where R, R′, R″ = H, alkyl, aryl, etc. They are a subset of chiral phosphines, a broader class of compounds where the stereogenic center can reside at sites other than phosphorus. P-chirality exploits the high barrier for inversion of phosphines, which ensures that enantiomers of PRR'R" do not racemize readily. The inversion barrier is relatively insensitive to substituents for triorganophosphines. [2] By contrast, most amines of the type NRR′R″ undergo rapid pyramidal inversion.
Most chiral phosphines are C2-symmetric diphosphines. Famous examples are DIPAMP and BINAP. These chelating ligands support catalysts used in asymmetric hydrogenation and related reactions. DIPAMP is prepared by coupling the P-chiral methylphenylanisylphosphine.
P-Chiral phosphines are of particular interest in asymmetric catalysis. P-Chiral phosphines have been investigated for two main applications, as ligands for asymmetric homogeneous catalysts and as nucleophiles in organocatalysis. [1]
- ^ a b Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. (2014). "Chiral Phosphines in Nucleophilic Organocatalysis". Beilstein Journal of Organic Chemistry. 10: 2089–2121. doi: 10.3762/bjoc.10.218. PMC 4168899. PMID 25246969.
- ^ Mislow, Kurt; Baechler, Raymond D. (1971). "Effect of ligand electronegativity on the inversion barrier of phosphines". Journal of the American Chemical Society. 93 (3): 773–774. doi: 10.1021/ja00732a036.

P-Chiral phosphines are organophosphorus compounds of the formula PRR′R″, where R, R′, R″ = H, alkyl, aryl, etc. They are a subset of chiral phosphines, a broader class of compounds where the stereogenic center can reside at sites other than phosphorus. P-chirality exploits the high barrier for inversion of phosphines, which ensures that enantiomers of PRR'R" do not racemize readily. The inversion barrier is relatively insensitive to substituents for triorganophosphines. [2] By contrast, most amines of the type NRR′R″ undergo rapid pyramidal inversion.
Most chiral phosphines are C2-symmetric diphosphines. Famous examples are DIPAMP and BINAP. These chelating ligands support catalysts used in asymmetric hydrogenation and related reactions. DIPAMP is prepared by coupling the P-chiral methylphenylanisylphosphine.
P-Chiral phosphines are of particular interest in asymmetric catalysis. P-Chiral phosphines have been investigated for two main applications, as ligands for asymmetric homogeneous catalysts and as nucleophiles in organocatalysis. [1]
- ^ a b Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. (2014). "Chiral Phosphines in Nucleophilic Organocatalysis". Beilstein Journal of Organic Chemistry. 10: 2089–2121. doi: 10.3762/bjoc.10.218. PMC 4168899. PMID 25246969.
- ^ Mislow, Kurt; Baechler, Raymond D. (1971). "Effect of ligand electronegativity on the inversion barrier of phosphines". Journal of the American Chemical Society. 93 (3): 773–774. doi: 10.1021/ja00732a036.