Optogenetics began with methods to alter neuronal activity with light, using e.g. channelrhodopsins. In a broader sense, optogenetic approaches also include the use of genetically encoded biosensors to monitor the activity of neurons or other cell types by measuring fluorescence or bioluminescence. Genetically encoded calcium indicators (GECIs) are used frequently to monitor neuronal activity, but other cellular parameters such as membrane voltage or second messenger activity can also be recorded optically. The use of optogenetic sensors is not restricted to neuroscience, but plays increasingly important roles in immunology, cardiology and cancer research.
History
The first experiments to measure intracellular calcium levels via protein expression were based on aequorin, a bioluminescent protein from the jellyfish Aequorea. To produce light, however, this enzyme needs the 'fuel' compound coelenteracine, which has to be added to the preparation. This is not practical in intact animals, and in addition, the temporal resolution of bioluminescence imaging is relatively poor (seconds-minutes). The first genetically encoded fluorescent calcium indicator (GECI) to be used to image activity in an animal was cameleon, designed by Atsushi Miyawaki, Roger Tsien and coworkers in 1997. [1] Cameleon was first used successfully in an animal by Rex Kerr, William Schafer and coworkers to record from neurons and muscle cells of the nematode C. elegans. [2] Cameleon was subsequently used to record neural activity in flies [3] and zebrafish. [4] In mammals, the first GECI to be used in vivo was GCaMP, [5] first developed by Junichi Nakai and coworkers in 2001. [6] GCaMP has undergone numerous improvements, notably by a team of scientists at the Janelia Farm Research Campus (GENIE project, HHMI), and GCaMP6 [7] in particular has become widely used in neuroscience. Very recently, G protein-coupled receptors have been harnessed to generate a series of highly specific indicators for various neurotransmitters. [8] [9]
Design principles
Genetically encoded sensors are fusion proteins, consisting of a ligand binding domain (sensor) and a fluorescent protein, attached by a short linker (flexible peptide). When the sensor domain binds the correct ligand, it changes conformation. This movement is transferred to the fluorescent protein and the resulting deformation leads to a change in fluorescence. The efficiency of this process depends critically on the length of the linker region, which has to be optimized in a labor-intensive process. The fluorescent protein is often circularly permuted, i.e. new C-terminal and N-terminal ends were created. Single-wavelength sensors are easy to use for qualitative measurements, but difficult to calibrate for quantitative measurements of ligand concentration.
A second class of sensors relies on Förster resonance energy transfer (FRET) between two fluorescent proteins (FP) of different color. The shorter wavelength FP (donor) is excited with blue light from a laser or LED. If the second FP (acceptor) is very close, the energy is transferred to the acceptor, resulting in yellow or red fluorescence. When the acceptor FP moves further away, the donor emits green fluorescence. The sensor domain is typically spliced between the two FPs, resulting in a hinge-type movement upon ligand binding that changes the distance between donor and acceptor. The imaging procedure is more complex for FRET sensors, but the fluorescence ratio can be calibrated to measure the absolute concentration of a ligand. Read-out via fluorescence lifetime imaging (FLIM) of donor fluorescence is also possible, as the FRET process speeds up the fluorescence decay.
Advantages of optogenetic sensors
- can be targeted to specific classes of cells (e.g. astrocytes or pyramidal cells). This allows for optical read-out without spatial resolution, e.g. fiber photometry from deep brain areas. [10]
- can be targeted to sub-cellular compartments (e.g. synapses, organelles, nucleus) by fusing the indicator protein with specific anchoring domains, retention signals or intrabodies.
- work in a variety of species ( nematodes, insects, fish, mammals) and in cell culture systems ( FLIPR assay)
- can be delivered by viral vectors (e.g. rAAV)
- can be used to record the activity of thousands of neurons at the same time [11]
Drawbacks, limitations
- will buffer the measured ion or protein, potentially interfering with cellular signaling
- are subject to photobleaching, compromising long-term measurements
- can be toxic when expressed at very high concentration
- require highly sensitive cameras or laser scanning microscopes
- some GPCR-based sensors are sensitive to polarization [12]
- most indicators are green fluorescent, making it difficult to measure several cellular parameters simultaneously ( multiplexing).
Classes of genetically encoded indicators
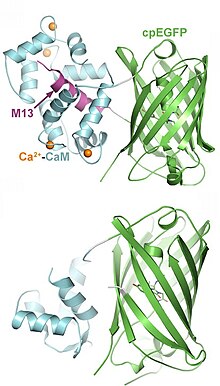
Indicators have been designed to measure ion concentrations, membrane potential, neurotransmitters, and various intracellular signaling molecules. The following list provides only examples for each class; many more have been published.
Intracellular signaling
- Genetically encoded calcium indicators (GECI): A large class of tools, based on natural calcium binding proteins ( calmodulin, troponin). Different affinities, kinetics, and colors (green, red) available. Read-out via fluorescence intensity (single wavelength indicators), FRET or BRET. Have been targeted to various organelles. Current version: JGCaMP8 [13]
- Genetically encoded chloride indicators: Clomeleon [14]
- Genetically encoded potassium indicators: GINKO2 [15]
- Genetically encoded indicators for intracellular pH (GEPhI): CypHer [16]
- Genetically encoded voltage indicators (GEVI): ArcLight [17]
- Genetically encoded vesicle fusion sensors: Synapto-pHluorin, Synaptophysin-pHluorin [18]
- Genetically encoded cAMP sensors: EPAC [19]
- Genetically encoded ATP sensors: QUEEN-37C [20]
- Genetically encoded kinase activity sensors: CaMui, [21] SmURFP
- Genetically encoded Small G-protein sensors: FRas [22]
Neurotransmitters and other extracellular signals
- Genetically encoded glutamate sensors: GluSnFR [23]
- Genetically encoded GABA sensors: iGABASnFR [24]
- Genetically encoded dopamine sensors: dLight1, [25] GRAB-DA [26]
- Genetically encoded serotonin sensors: GRAB5-HT, [27] sDarken, [28] iSeroSnFR [29]
- Genetically encoded norepinephrine sensors: GRABNE [30]
- Genetically encoded sensor for endocannabinoid activity: GRABeCB2.0 [31]
- Genetically encoded sensor for orexin/hypocretin neuropeptides: OxLight1 [32]
- Genetically encoded sensor for lactate: eLACCO1.1 [33]
Further reading
A recent review of GPCR-based genetically encoded fluorescent indicators for neuromodulators [9]
External links
- Fluorescent Biosensor Database, a fairly complete searchable list of published sensors and their basic properties, maintained by Jin Zhang's lab at UCSD. [34]
- Fluorescent Biosensors available on Addgene, a nonprofit plasmid repository.
References
- ^ Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (August 1997). "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature. 388 (6645): 882–887. Bibcode: 1997Natur.388..882M. doi: 10.1038/42264. PMID 9278050. S2CID 13745050.
- ^ Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR (June 2000). "Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans". Neuron. 26 (3): 583–594. doi: 10.1016/s0896-6273(00)81196-4. PMID 10896155. S2CID 311998.
- ^ Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, et al. (October 2002). "Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons". Current Biology. 12 (21): 1877–1884. Bibcode: 2002CBio...12.1877F. doi: 10.1016/s0960-9822(02)01239-3. PMID 12419190. S2CID 6312049.
- ^ Higashijima S, Masino MA, Mandel G, Fetcho JR (December 2003). "Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator". Journal of Neurophysiology. 90 (6): 3986–3997. doi: 10.1152/jn.00576.2003. PMID 12930818. S2CID 2230173.
- ^ Ji G, Feldman ME, Deng KY, Greene KS, Wilson J, Lee JC, et al. (May 2004). "Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle". The Journal of Biological Chemistry. 279 (20): 21461–21468. doi: 10.1074/jbc.M401084200. PMID 14990564.
- ^ Nakai J, Ohkura M, Imoto K (February 2001). "A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein". Nature Biotechnology. 19 (2): 137–141. doi: 10.1038/84397. PMID 11175727. S2CID 30254550.
- ^ Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. (July 2013). "Ultrasensitive fluorescent proteins for imaging neuronal activity". Nature. 499 (7458): 295–300. Bibcode: 2013Natur.499..295C. doi: 10.1038/nature12354. PMC 3777791. PMID 23868258.
- ^ Ravotto L, Duffet L, Zhou X, Weber B, Patriarchi T (2020). "A Bright and Colorful Future for G-Protein Coupled Receptor Sensors". Frontiers in Cellular Neuroscience. 14: 67. doi: 10.3389/fncel.2020.00067. PMC 7098945. PMID 32265667.
- ^ a b Rohner, Valentin Lu; Lamothe-Molina, Paul J.; Patriarchi, Tommaso (2024-01-30). "Engineering, applications, and future perspectives of GPCR -based genetically encoded fluorescent indicators for neuromodulators". Journal of Neurochemistry. 168 (3): 163–184. doi: 10.1111/jnc.16045. hdl: 20.500.11850/659388. ISSN 0022-3042. PMID 38288673.
- ^ Jones-Tabah J, Mohammad H, Hadj-Youssef S, Kim LE, Martin RD, Benaliouad F, et al. (September 2020). "Dopamine D1 receptor signalling in dyskinetic Parkinsonian rats revealed by fiber photometry using FRET-based biosensors". Scientific Reports. 10 (1): 14426. Bibcode: 2020NatSR..1014426J. doi: 10.1038/s41598-020-71121-8. PMC 7468292. PMID 32879346.
- ^ Sofroniew NJ (September 2017). "Q&A: The brain under a mesoscope: the forest and the trees". BMC Biology. 15 (1): 82. doi: 10.1186/s12915-017-0426-y. PMC 5598035. PMID 28911321.
- ^ Pulin M, Stockhausen KE, Masseck OA, Kubitschke M, Busse B, Wiegert JS, Oertner TG (February 2022). "Orthogonally-polarized excitation for improved two-photon and second-harmonic-generation microscopy, applied to neurotransmitter imaging with GPCR-based sensors". Biomedical Optics Express. 13 (2): 777–790. doi: 10.1364/BOE.448760. PMC 8884218. PMID 35284188.
- ^ Zhang Y, Rózsa M, Bushey D, Zheng J, Reep D, Broussard GJ, Tsang A, Tsegaye G, Patel R, Narayan S, Lim JX (2020). "jGCaMP8 Fast Genetically Encoded Calcium Indicators". Janelia Research Campus: 361685. doi: 10.25378/JANELIA.13148243.
- ^ Berglund K, Schleich W, Wang H, Feng G, Hall WC, Kuner T, Augustine GJ (August 2008). "Imaging synaptic inhibition throughout the brain via genetically targeted Clomeleon". Brain Cell Biology. 36 (1–4): 101–118. doi: 10.1007/s11068-008-9031-x. PMC 2674236. PMID 18850274.
- ^ Wu, Sheng-Yi; Wen, Yurong; Serre, Nelson B. C.; Laursen, Cathrine Charlotte Heiede; Dietz, Andrea Grostøl; Taylor, Brian R.; Drobizhev, Mikhail; Molina, Rosana S.; Aggarwal, Abhi; Rancic, Vladimir; Becker, Michael; Ballanyi, Klaus; Podgorski, Kaspar; Hirase, Hajime; Nedergaard, Maiken (2022-09-06). Dutzler, Raimund (ed.). "A sensitive and specific genetically-encoded potassium ion biosensor for in vivo applications across the tree of life". PLOS Biology. 20 (9): e3001772. doi: 10.1371/journal.pbio.3001772. ISSN 1545-7885. PMC 9481166. PMID 36067248.
- ^ Han J, Burgess K (May 2010). "Fluorescent indicators for intracellular pH". Chemical Reviews. 110 (5): 2709–2728. doi: 10.1021/cr900249z. PMID 19831417.
- ^ Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA (September 2012). "Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe". Neuron. 75 (5): 779–785. doi: 10.1016/j.neuron.2012.06.040. PMC 3439164. PMID 22958819.
- ^ Granseth B, Odermatt B, Royle SJ, Lagnado L (September 2006). "Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses". Neuron. 51 (6): 773–786. doi: 10.1016/j.neuron.2006.08.029. PMID 16982422. S2CID 921124.
- ^ Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K (2015-04-14). "Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity". PLOS ONE. 10 (4): e0122513. Bibcode: 2015PLoSO..1022513K. doi: 10.1371/journal.pone.0122513. PMC 4397040. PMID 25875503.
- ^ Yaginuma H, Okada Y (2021-10-09). "Live cell imaging of metabolic heterogeneity by quantitative fluorescent ATP indicator protein, QUEEN-37C". bioRxiv: 2021.10.08.463131. doi: 10.1101/2021.10.08.463131. S2CID 238585891.
- ^ Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (March 2009). "Activation of CaMKII in single dendritic spines during long-term potentiation". Nature. 458 (7236): 299–304. Bibcode: 2009Natur.458..299L. doi: 10.1038/nature07842. PMC 2719773. PMID 19295602.
- ^ Oliveira AF, Yasuda R (2013-01-14). "An improved Ras sensor for highly sensitive and quantitative FRET-FLIM imaging". PLOS ONE. 8 (1): e52874. Bibcode: 2013PLoSO...852874O. doi: 10.1371/journal.pone.0052874. PMC 3544822. PMID 23349692.
- ^ Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Müller JA, et al. (November 2018). "Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR". Nature Methods. 15 (11): 936–939. doi: 10.1038/s41592-018-0171-3. PMC 6394230. PMID 30377363.
- ^ Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, et al. (August 2019). "A genetically encoded fluorescent sensor for in vivo imaging of GABA". Nature Methods. 16 (8): 763–770. doi: 10.1038/s41592-019-0471-2. PMID 31308547. S2CID 196812412.
- ^ Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, et al. (June 2018). "Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors". Science. 360 (6396): eaat4422. doi: 10.1126/science.aat4422. PMC 6287765. PMID 29853555.
- ^ Labouesse MA, Cola RB, Patriarchi T (October 2020). "GPCR-Based Dopamine Sensors-A Detailed Guide to Inform Sensor Choice for In vivo Imaging". International Journal of Molecular Sciences. 21 (21): 8048. doi: 10.3390/ijms21218048. PMC 7672611. PMID 33126757.
- ^ Wan J, Peng W, Li X, Qian T, Song K, Zeng J, et al. (May 2021). "A genetically encoded sensor for measuring serotonin dynamics". Nature Neuroscience. 24 (5): 746–752. doi: 10.1038/s41593-021-00823-7. PMC 8544647. PMID 33821000.
- ^ Kubitschke, Martin; Müller, Monika; Wallhorn, Lutz; Pulin, Mauro; Mittag, Manuel; Pollok, Stefan; Ziebarth, Tim; Bremshey, Svenja; Gerdey, Jill; Claussen, Kristin Carolin; Renken, Kim; Groß, Juliana; Gneiße, Pascal; Meyer, Niklas; Wiegert, J. Simon (2022-12-06). "Next generation genetically encoded fluorescent sensors for serotonin". Nature Communications. 13 (1): 7525. Bibcode: 2022NatCo..13.7525K. doi: 10.1038/s41467-022-35200-w. ISSN 2041-1723. PMC 9726753. PMID 36473867. S2CID 247454046.
- ^ Unger, Elizabeth K.; Keller, Jacob P.; Altermatt, Michael; Liang, Ruqiang; Matsui, Aya; et al. (December 2020). "Directed Evolution of a Selective and Sensitive Serotonin Sensor via Machine Learning". Cell. 183 (7): 1986–2002.e26. doi: 10.1016/j.cell.2020.11.040. PMC 8025677. PMID 33333022.
- ^ Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, et al. (May 2019). "A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine". Neuron. 102 (4): 745–761.e8. doi: 10.1016/j.neuron.2019.02.037. PMC 6533151. PMID 30922875.
- ^ Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, et al. (November 2021). "A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo". Nature Biotechnology. 40 (5): 787–798. doi: 10.1038/s41587-021-01074-4. PMC 9091059. PMID 34764491. S2CID 244039925.
- ^ Duffet L, Kosar S, Panniello M, Viberti B, Bracey E, Zych AD, et al. (February 2022). "A genetically encoded sensor for in vivo imaging of orexin neuropeptides". Nature Methods. 19 (2): 231–241. doi: 10.1038/s41592-021-01390-2. PMC 8831244. PMID 35145320.
- ^ Nasu, Yusuke; Murphy-Royal, Ciaran; Wen, Yurong; Haidey, Jordan N.; Molina, Rosana S.; Aggarwal, Abhi; Zhang, Shuce; Kamijo, Yuki; Paquet, Marie-Eve; Podgorski, Kaspar; Drobizhev, Mikhail; Bains, Jaideep S.; Lemieux, M. Joanne; Gordon, Grant R.; Campbell, Robert E. (2021-12-06). "A genetically encoded fluorescent biosensor for extracellular l-lactate". Nature Communications. 12 (1): 7058. Bibcode: 2021NatCo..12.7058N. doi: 10.1038/s41467-021-27332-2. ISSN 2041-1723. PMC 8648760. PMID 34873165.
- ^ Greenwald EC, Mehta S, Zhang J (December 2018). "Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks". Chemical Reviews. 118 (24): 11707–11794. doi: 10.1021/acs.chemrev.8b00333. PMC 7462118. PMID 30550275.
Optogenetics began with methods to alter neuronal activity with light, using e.g. channelrhodopsins. In a broader sense, optogenetic approaches also include the use of genetically encoded biosensors to monitor the activity of neurons or other cell types by measuring fluorescence or bioluminescence. Genetically encoded calcium indicators (GECIs) are used frequently to monitor neuronal activity, but other cellular parameters such as membrane voltage or second messenger activity can also be recorded optically. The use of optogenetic sensors is not restricted to neuroscience, but plays increasingly important roles in immunology, cardiology and cancer research.
History
The first experiments to measure intracellular calcium levels via protein expression were based on aequorin, a bioluminescent protein from the jellyfish Aequorea. To produce light, however, this enzyme needs the 'fuel' compound coelenteracine, which has to be added to the preparation. This is not practical in intact animals, and in addition, the temporal resolution of bioluminescence imaging is relatively poor (seconds-minutes). The first genetically encoded fluorescent calcium indicator (GECI) to be used to image activity in an animal was cameleon, designed by Atsushi Miyawaki, Roger Tsien and coworkers in 1997. [1] Cameleon was first used successfully in an animal by Rex Kerr, William Schafer and coworkers to record from neurons and muscle cells of the nematode C. elegans. [2] Cameleon was subsequently used to record neural activity in flies [3] and zebrafish. [4] In mammals, the first GECI to be used in vivo was GCaMP, [5] first developed by Junichi Nakai and coworkers in 2001. [6] GCaMP has undergone numerous improvements, notably by a team of scientists at the Janelia Farm Research Campus (GENIE project, HHMI), and GCaMP6 [7] in particular has become widely used in neuroscience. Very recently, G protein-coupled receptors have been harnessed to generate a series of highly specific indicators for various neurotransmitters. [8] [9]
Design principles
Genetically encoded sensors are fusion proteins, consisting of a ligand binding domain (sensor) and a fluorescent protein, attached by a short linker (flexible peptide). When the sensor domain binds the correct ligand, it changes conformation. This movement is transferred to the fluorescent protein and the resulting deformation leads to a change in fluorescence. The efficiency of this process depends critically on the length of the linker region, which has to be optimized in a labor-intensive process. The fluorescent protein is often circularly permuted, i.e. new C-terminal and N-terminal ends were created. Single-wavelength sensors are easy to use for qualitative measurements, but difficult to calibrate for quantitative measurements of ligand concentration.
A second class of sensors relies on Förster resonance energy transfer (FRET) between two fluorescent proteins (FP) of different color. The shorter wavelength FP (donor) is excited with blue light from a laser or LED. If the second FP (acceptor) is very close, the energy is transferred to the acceptor, resulting in yellow or red fluorescence. When the acceptor FP moves further away, the donor emits green fluorescence. The sensor domain is typically spliced between the two FPs, resulting in a hinge-type movement upon ligand binding that changes the distance between donor and acceptor. The imaging procedure is more complex for FRET sensors, but the fluorescence ratio can be calibrated to measure the absolute concentration of a ligand. Read-out via fluorescence lifetime imaging (FLIM) of donor fluorescence is also possible, as the FRET process speeds up the fluorescence decay.
Advantages of optogenetic sensors
- can be targeted to specific classes of cells (e.g. astrocytes or pyramidal cells). This allows for optical read-out without spatial resolution, e.g. fiber photometry from deep brain areas. [10]
- can be targeted to sub-cellular compartments (e.g. synapses, organelles, nucleus) by fusing the indicator protein with specific anchoring domains, retention signals or intrabodies.
- work in a variety of species ( nematodes, insects, fish, mammals) and in cell culture systems ( FLIPR assay)
- can be delivered by viral vectors (e.g. rAAV)
- can be used to record the activity of thousands of neurons at the same time [11]
Drawbacks, limitations
- will buffer the measured ion or protein, potentially interfering with cellular signaling
- are subject to photobleaching, compromising long-term measurements
- can be toxic when expressed at very high concentration
- require highly sensitive cameras or laser scanning microscopes
- some GPCR-based sensors are sensitive to polarization [12]
- most indicators are green fluorescent, making it difficult to measure several cellular parameters simultaneously ( multiplexing).
Classes of genetically encoded indicators

Indicators have been designed to measure ion concentrations, membrane potential, neurotransmitters, and various intracellular signaling molecules. The following list provides only examples for each class; many more have been published.
Intracellular signaling
- Genetically encoded calcium indicators (GECI): A large class of tools, based on natural calcium binding proteins ( calmodulin, troponin). Different affinities, kinetics, and colors (green, red) available. Read-out via fluorescence intensity (single wavelength indicators), FRET or BRET. Have been targeted to various organelles. Current version: JGCaMP8 [13]
- Genetically encoded chloride indicators: Clomeleon [14]
- Genetically encoded potassium indicators: GINKO2 [15]
- Genetically encoded indicators for intracellular pH (GEPhI): CypHer [16]
- Genetically encoded voltage indicators (GEVI): ArcLight [17]
- Genetically encoded vesicle fusion sensors: Synapto-pHluorin, Synaptophysin-pHluorin [18]
- Genetically encoded cAMP sensors: EPAC [19]
- Genetically encoded ATP sensors: QUEEN-37C [20]
- Genetically encoded kinase activity sensors: CaMui, [21] SmURFP
- Genetically encoded Small G-protein sensors: FRas [22]
Neurotransmitters and other extracellular signals
- Genetically encoded glutamate sensors: GluSnFR [23]
- Genetically encoded GABA sensors: iGABASnFR [24]
- Genetically encoded dopamine sensors: dLight1, [25] GRAB-DA [26]
- Genetically encoded serotonin sensors: GRAB5-HT, [27] sDarken, [28] iSeroSnFR [29]
- Genetically encoded norepinephrine sensors: GRABNE [30]
- Genetically encoded sensor for endocannabinoid activity: GRABeCB2.0 [31]
- Genetically encoded sensor for orexin/hypocretin neuropeptides: OxLight1 [32]
- Genetically encoded sensor for lactate: eLACCO1.1 [33]
Further reading
A recent review of GPCR-based genetically encoded fluorescent indicators for neuromodulators [9]
External links
- Fluorescent Biosensor Database, a fairly complete searchable list of published sensors and their basic properties, maintained by Jin Zhang's lab at UCSD. [34]
- Fluorescent Biosensors available on Addgene, a nonprofit plasmid repository.
References
- ^ Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (August 1997). "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature. 388 (6645): 882–887. Bibcode: 1997Natur.388..882M. doi: 10.1038/42264. PMID 9278050. S2CID 13745050.
- ^ Kerr R, Lev-Ram V, Baird G, Vincent P, Tsien RY, Schafer WR (June 2000). "Optical imaging of calcium transients in neurons and pharyngeal muscle of C. elegans". Neuron. 26 (3): 583–594. doi: 10.1016/s0896-6273(00)81196-4. PMID 10896155. S2CID 311998.
- ^ Fiala A, Spall T, Diegelmann S, Eisermann B, Sachse S, Devaud JM, et al. (October 2002). "Genetically expressed cameleon in Drosophila melanogaster is used to visualize olfactory information in projection neurons". Current Biology. 12 (21): 1877–1884. Bibcode: 2002CBio...12.1877F. doi: 10.1016/s0960-9822(02)01239-3. PMID 12419190. S2CID 6312049.
- ^ Higashijima S, Masino MA, Mandel G, Fetcho JR (December 2003). "Imaging neuronal activity during zebrafish behavior with a genetically encoded calcium indicator". Journal of Neurophysiology. 90 (6): 3986–3997. doi: 10.1152/jn.00576.2003. PMID 12930818. S2CID 2230173.
- ^ Ji G, Feldman ME, Deng KY, Greene KS, Wilson J, Lee JC, et al. (May 2004). "Ca2+-sensing transgenic mice: postsynaptic signaling in smooth muscle". The Journal of Biological Chemistry. 279 (20): 21461–21468. doi: 10.1074/jbc.M401084200. PMID 14990564.
- ^ Nakai J, Ohkura M, Imoto K (February 2001). "A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein". Nature Biotechnology. 19 (2): 137–141. doi: 10.1038/84397. PMID 11175727. S2CID 30254550.
- ^ Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, et al. (July 2013). "Ultrasensitive fluorescent proteins for imaging neuronal activity". Nature. 499 (7458): 295–300. Bibcode: 2013Natur.499..295C. doi: 10.1038/nature12354. PMC 3777791. PMID 23868258.
- ^ Ravotto L, Duffet L, Zhou X, Weber B, Patriarchi T (2020). "A Bright and Colorful Future for G-Protein Coupled Receptor Sensors". Frontiers in Cellular Neuroscience. 14: 67. doi: 10.3389/fncel.2020.00067. PMC 7098945. PMID 32265667.
- ^ a b Rohner, Valentin Lu; Lamothe-Molina, Paul J.; Patriarchi, Tommaso (2024-01-30). "Engineering, applications, and future perspectives of GPCR -based genetically encoded fluorescent indicators for neuromodulators". Journal of Neurochemistry. 168 (3): 163–184. doi: 10.1111/jnc.16045. hdl: 20.500.11850/659388. ISSN 0022-3042. PMID 38288673.
- ^ Jones-Tabah J, Mohammad H, Hadj-Youssef S, Kim LE, Martin RD, Benaliouad F, et al. (September 2020). "Dopamine D1 receptor signalling in dyskinetic Parkinsonian rats revealed by fiber photometry using FRET-based biosensors". Scientific Reports. 10 (1): 14426. Bibcode: 2020NatSR..1014426J. doi: 10.1038/s41598-020-71121-8. PMC 7468292. PMID 32879346.
- ^ Sofroniew NJ (September 2017). "Q&A: The brain under a mesoscope: the forest and the trees". BMC Biology. 15 (1): 82. doi: 10.1186/s12915-017-0426-y. PMC 5598035. PMID 28911321.
- ^ Pulin M, Stockhausen KE, Masseck OA, Kubitschke M, Busse B, Wiegert JS, Oertner TG (February 2022). "Orthogonally-polarized excitation for improved two-photon and second-harmonic-generation microscopy, applied to neurotransmitter imaging with GPCR-based sensors". Biomedical Optics Express. 13 (2): 777–790. doi: 10.1364/BOE.448760. PMC 8884218. PMID 35284188.
- ^ Zhang Y, Rózsa M, Bushey D, Zheng J, Reep D, Broussard GJ, Tsang A, Tsegaye G, Patel R, Narayan S, Lim JX (2020). "jGCaMP8 Fast Genetically Encoded Calcium Indicators". Janelia Research Campus: 361685. doi: 10.25378/JANELIA.13148243.
- ^ Berglund K, Schleich W, Wang H, Feng G, Hall WC, Kuner T, Augustine GJ (August 2008). "Imaging synaptic inhibition throughout the brain via genetically targeted Clomeleon". Brain Cell Biology. 36 (1–4): 101–118. doi: 10.1007/s11068-008-9031-x. PMC 2674236. PMID 18850274.
- ^ Wu, Sheng-Yi; Wen, Yurong; Serre, Nelson B. C.; Laursen, Cathrine Charlotte Heiede; Dietz, Andrea Grostøl; Taylor, Brian R.; Drobizhev, Mikhail; Molina, Rosana S.; Aggarwal, Abhi; Rancic, Vladimir; Becker, Michael; Ballanyi, Klaus; Podgorski, Kaspar; Hirase, Hajime; Nedergaard, Maiken (2022-09-06). Dutzler, Raimund (ed.). "A sensitive and specific genetically-encoded potassium ion biosensor for in vivo applications across the tree of life". PLOS Biology. 20 (9): e3001772. doi: 10.1371/journal.pbio.3001772. ISSN 1545-7885. PMC 9481166. PMID 36067248.
- ^ Han J, Burgess K (May 2010). "Fluorescent indicators for intracellular pH". Chemical Reviews. 110 (5): 2709–2728. doi: 10.1021/cr900249z. PMID 19831417.
- ^ Jin L, Han Z, Platisa J, Wooltorton JR, Cohen LB, Pieribone VA (September 2012). "Single action potentials and subthreshold electrical events imaged in neurons with a fluorescent protein voltage probe". Neuron. 75 (5): 779–785. doi: 10.1016/j.neuron.2012.06.040. PMC 3439164. PMID 22958819.
- ^ Granseth B, Odermatt B, Royle SJ, Lagnado L (September 2006). "Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses". Neuron. 51 (6): 773–786. doi: 10.1016/j.neuron.2006.08.029. PMID 16982422. S2CID 921124.
- ^ Klarenbeek J, Goedhart J, van Batenburg A, Groenewald D, Jalink K (2015-04-14). "Fourth-generation epac-based FRET sensors for cAMP feature exceptional brightness, photostability and dynamic range: characterization of dedicated sensors for FLIM, for ratiometry and with high affinity". PLOS ONE. 10 (4): e0122513. Bibcode: 2015PLoSO..1022513K. doi: 10.1371/journal.pone.0122513. PMC 4397040. PMID 25875503.
- ^ Yaginuma H, Okada Y (2021-10-09). "Live cell imaging of metabolic heterogeneity by quantitative fluorescent ATP indicator protein, QUEEN-37C". bioRxiv: 2021.10.08.463131. doi: 10.1101/2021.10.08.463131. S2CID 238585891.
- ^ Lee SJ, Escobedo-Lozoya Y, Szatmari EM, Yasuda R (March 2009). "Activation of CaMKII in single dendritic spines during long-term potentiation". Nature. 458 (7236): 299–304. Bibcode: 2009Natur.458..299L. doi: 10.1038/nature07842. PMC 2719773. PMID 19295602.
- ^ Oliveira AF, Yasuda R (2013-01-14). "An improved Ras sensor for highly sensitive and quantitative FRET-FLIM imaging". PLOS ONE. 8 (1): e52874. Bibcode: 2013PLoSO...852874O. doi: 10.1371/journal.pone.0052874. PMC 3544822. PMID 23349692.
- ^ Marvin JS, Scholl B, Wilson DE, Podgorski K, Kazemipour A, Müller JA, et al. (November 2018). "Stability, affinity, and chromatic variants of the glutamate sensor iGluSnFR". Nature Methods. 15 (11): 936–939. doi: 10.1038/s41592-018-0171-3. PMC 6394230. PMID 30377363.
- ^ Marvin JS, Shimoda Y, Magloire V, Leite M, Kawashima T, Jensen TP, et al. (August 2019). "A genetically encoded fluorescent sensor for in vivo imaging of GABA". Nature Methods. 16 (8): 763–770. doi: 10.1038/s41592-019-0471-2. PMID 31308547. S2CID 196812412.
- ^ Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong WH, et al. (June 2018). "Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors". Science. 360 (6396): eaat4422. doi: 10.1126/science.aat4422. PMC 6287765. PMID 29853555.
- ^ Labouesse MA, Cola RB, Patriarchi T (October 2020). "GPCR-Based Dopamine Sensors-A Detailed Guide to Inform Sensor Choice for In vivo Imaging". International Journal of Molecular Sciences. 21 (21): 8048. doi: 10.3390/ijms21218048. PMC 7672611. PMID 33126757.
- ^ Wan J, Peng W, Li X, Qian T, Song K, Zeng J, et al. (May 2021). "A genetically encoded sensor for measuring serotonin dynamics". Nature Neuroscience. 24 (5): 746–752. doi: 10.1038/s41593-021-00823-7. PMC 8544647. PMID 33821000.
- ^ Kubitschke, Martin; Müller, Monika; Wallhorn, Lutz; Pulin, Mauro; Mittag, Manuel; Pollok, Stefan; Ziebarth, Tim; Bremshey, Svenja; Gerdey, Jill; Claussen, Kristin Carolin; Renken, Kim; Groß, Juliana; Gneiße, Pascal; Meyer, Niklas; Wiegert, J. Simon (2022-12-06). "Next generation genetically encoded fluorescent sensors for serotonin". Nature Communications. 13 (1): 7525. Bibcode: 2022NatCo..13.7525K. doi: 10.1038/s41467-022-35200-w. ISSN 2041-1723. PMC 9726753. PMID 36473867. S2CID 247454046.
- ^ Unger, Elizabeth K.; Keller, Jacob P.; Altermatt, Michael; Liang, Ruqiang; Matsui, Aya; et al. (December 2020). "Directed Evolution of a Selective and Sensitive Serotonin Sensor via Machine Learning". Cell. 183 (7): 1986–2002.e26. doi: 10.1016/j.cell.2020.11.040. PMC 8025677. PMID 33333022.
- ^ Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, et al. (May 2019). "A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine". Neuron. 102 (4): 745–761.e8. doi: 10.1016/j.neuron.2019.02.037. PMC 6533151. PMID 30922875.
- ^ Dong A, He K, Dudok B, Farrell JS, Guan W, Liput DJ, et al. (November 2021). "A fluorescent sensor for spatiotemporally resolved imaging of endocannabinoid dynamics in vivo". Nature Biotechnology. 40 (5): 787–798. doi: 10.1038/s41587-021-01074-4. PMC 9091059. PMID 34764491. S2CID 244039925.
- ^ Duffet L, Kosar S, Panniello M, Viberti B, Bracey E, Zych AD, et al. (February 2022). "A genetically encoded sensor for in vivo imaging of orexin neuropeptides". Nature Methods. 19 (2): 231–241. doi: 10.1038/s41592-021-01390-2. PMC 8831244. PMID 35145320.
- ^ Nasu, Yusuke; Murphy-Royal, Ciaran; Wen, Yurong; Haidey, Jordan N.; Molina, Rosana S.; Aggarwal, Abhi; Zhang, Shuce; Kamijo, Yuki; Paquet, Marie-Eve; Podgorski, Kaspar; Drobizhev, Mikhail; Bains, Jaideep S.; Lemieux, M. Joanne; Gordon, Grant R.; Campbell, Robert E. (2021-12-06). "A genetically encoded fluorescent biosensor for extracellular l-lactate". Nature Communications. 12 (1): 7058. Bibcode: 2021NatCo..12.7058N. doi: 10.1038/s41467-021-27332-2. ISSN 2041-1723. PMC 8648760. PMID 34873165.
- ^ Greenwald EC, Mehta S, Zhang J (December 2018). "Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks". Chemical Reviews. 118 (24): 11707–11794. doi: 10.1021/acs.chemrev.8b00333. PMC 7462118. PMID 30550275.