|
Structural formula of octamethylenediamine
| |
| Names | |
|---|---|
|
IUPAC name
Octane-1,8-diamine
| |
Other names
| |
| Identifiers | |
3D model (
JSmol)
|
|
| Abbreviations | OMDA |
| 3-04-00-00612 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.150 |
| EC Number |
|
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C8H20N2 | |
| Molar mass | 144.26 g mol−1 |
| Appearance | colorless solid with an amine-like odor [1] |
| Density | 0.83 g cm−3 (60 °C) [1] |
| Melting point | 52 °C [3] |
| Boiling point | 225-226 °C [1] [2] |
| Easily soluble in water (575 g l−1 at 20°C) [1] | |
| Vapor pressure | |
| Hazards | |
| GHS labelling: [1] | |


| |
| Danger | |
| H302, H314, H317 | |
| P260, P280, P301+P312+P330, P303+P361+P353, P305+P351+P338+P310 | |
| Flash point | 113 °C |
| Safety data sheet (SDS) | "1,8-Diaminooctane". GESTIS-Stoffdatenbank. Retrieved 2022-08-05. |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Octamethylenediamine (OMDA) is an organic chemical compound from the substance group of aliphatic diamines. It is used as a versatile reaction intermediate in the manufacture of pesticides, especially fungicides.
Manufacture
The industrial production of octamethylene diamine is carried out by the catalytic hydrogenation of suberonitrile at temperatures of 150 to 180 °C and a pressure of 50 to 180 bar in the presence of ammonia over heterogeneous cobalt unsupported catalysts:
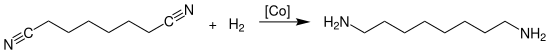
The reaction is carried out in the liquid phase and is carried out continuously or batchwise. The catalyst is arranged as a fixed bed in a shaft, tube, or tube bundle reactor.
Characteristics
Octamethylenediamine is a combustible but difficult to ignite. It is a solid that is easily soluble in water. The aqueous solutions are strongly alkaline ( pH value of 12.1 at a concentration of 10 g/L). [4]
Use
Octamethylenediamine is used as a versatile intermediate in manufacturing pesticides, especially fungicides. [5]
Safety instructions
While octamethylenediamine is combustible, it is difficult to ignite because it is solid at moderate temperatures. It has a lower explosive limit (LEL) of 1.1 % by volume and an upper explosive limit (UEL) of 6.8 % by volume. The ignition temperature is 280 °C The substance therefore falls into temperature class T3. With a flash point of 113 °C, the liquid is considered difficult to ignite. [4]
References
- ^ a b c d e f g h Record of Octamethylenediamine in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2022-01-20.
- ^ "373-44-4 - 1,8-Diaminooctane, 98% - 1,8-Octanediamine - B23885". Alfa Aesar. 2017-10-22. Retrieved 2022-08-06.
- ^ "1,8-Octanediamine". CAS Common Chemistry. Retrieved 2022-08-05.
- ^ a b "WO2018050555A1 Method for the Preparation of Polyamines from Dinitriles and/or Amino Nitrile". Espacenet – patent search. 2021-03-20. Retrieved 2022-08-05.
- ^ Peter Roose, Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke: Amines, Aliphatic. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag GmbH & Co. KGaA., 30. September 2015, doi: 10.1002/14356007.a02_001.pub2.
External links
- "1,8-diaminooctane". MetaCyc. 2022-08-05. Retrieved 2022-08-06.
- Thalladi, V.R.; Boese, R.; Weiss, H.-C. (2001), CCDC 132875: Experimental Crystal Structure Determination, Cambridge Crystallographic Data Centre, doi: 10.5517/CC4G894, retrieved 2022-08-06
|
Structural formula of octamethylenediamine
| |
| Names | |
|---|---|
|
IUPAC name
Octane-1,8-diamine
| |
Other names
| |
| Identifiers | |
3D model (
JSmol)
|
|
| Abbreviations | OMDA |
| 3-04-00-00612 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.006.150 |
| EC Number |
|
PubChem
CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C8H20N2 | |
| Molar mass | 144.26 g mol−1 |
| Appearance | colorless solid with an amine-like odor [1] |
| Density | 0.83 g cm−3 (60 °C) [1] |
| Melting point | 52 °C [3] |
| Boiling point | 225-226 °C [1] [2] |
| Easily soluble in water (575 g l−1 at 20°C) [1] | |
| Vapor pressure | |
| Hazards | |
| GHS labelling: [1] | |


| |
| Danger | |
| H302, H314, H317 | |
| P260, P280, P301+P312+P330, P303+P361+P353, P305+P351+P338+P310 | |
| Flash point | 113 °C |
| Safety data sheet (SDS) | "1,8-Diaminooctane". GESTIS-Stoffdatenbank. Retrieved 2022-08-05. |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Octamethylenediamine (OMDA) is an organic chemical compound from the substance group of aliphatic diamines. It is used as a versatile reaction intermediate in the manufacture of pesticides, especially fungicides.
Manufacture
The industrial production of octamethylene diamine is carried out by the catalytic hydrogenation of suberonitrile at temperatures of 150 to 180 °C and a pressure of 50 to 180 bar in the presence of ammonia over heterogeneous cobalt unsupported catalysts:

The reaction is carried out in the liquid phase and is carried out continuously or batchwise. The catalyst is arranged as a fixed bed in a shaft, tube, or tube bundle reactor.
Characteristics
Octamethylenediamine is a combustible but difficult to ignite. It is a solid that is easily soluble in water. The aqueous solutions are strongly alkaline ( pH value of 12.1 at a concentration of 10 g/L). [4]
Use
Octamethylenediamine is used as a versatile intermediate in manufacturing pesticides, especially fungicides. [5]
Safety instructions
While octamethylenediamine is combustible, it is difficult to ignite because it is solid at moderate temperatures. It has a lower explosive limit (LEL) of 1.1 % by volume and an upper explosive limit (UEL) of 6.8 % by volume. The ignition temperature is 280 °C The substance therefore falls into temperature class T3. With a flash point of 113 °C, the liquid is considered difficult to ignite. [4]
References
- ^ a b c d e f g h Record of Octamethylenediamine in the GESTIS Substance Database of the Institute for Occupational Safety and Health, accessed on 2022-01-20.
- ^ "373-44-4 - 1,8-Diaminooctane, 98% - 1,8-Octanediamine - B23885". Alfa Aesar. 2017-10-22. Retrieved 2022-08-06.
- ^ "1,8-Octanediamine". CAS Common Chemistry. Retrieved 2022-08-05.
- ^ a b "WO2018050555A1 Method for the Preparation of Polyamines from Dinitriles and/or Amino Nitrile". Espacenet – patent search. 2021-03-20. Retrieved 2022-08-05.
- ^ Peter Roose, Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke: Amines, Aliphatic. In: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley‐VCH Verlag GmbH & Co. KGaA., 30. September 2015, doi: 10.1002/14356007.a02_001.pub2.
External links
- "1,8-diaminooctane". MetaCyc. 2022-08-05. Retrieved 2022-08-06.
- Thalladi, V.R.; Boese, R.; Weiss, H.-C. (2001), CCDC 132875: Experimental Crystal Structure Determination, Cambridge Crystallographic Data Centre, doi: 10.5517/CC4G894, retrieved 2022-08-06