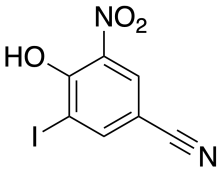
 Structure of nitroxinil | |
| Clinical data | |
|---|---|
| Trade names | Fluconix, Dovenix, Trodax |
| Other names | Nitroxynil |
|
Routes of administration | Subcutaneous in the form of an N-Ethylglucamine salt solution |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.015.350 |
| Chemical and physical data | |
| Formula | C7H3IN2O3 |
| Molar mass | 290.016 g·mol−1 |
| 3D model ( JSmol) | |
| Melting point | 136–139 °C (277–282 °F) |
| |
| |
Nitroxinil is an anthelmintic, a veterinary medicine against parasitic worms in sheep and cattle. The substance is active against the liver fluke the Fasciola hepatica and to a lesser extent against thread worms in the gastrointestinal tract. [1] Brand names include Fluconix, Dovenix and Trodax. Nitroxynil is also used against strains of the red gum worm ( Haemonchus contortus) that have become resistant to benzimidazoles.[ citation needed]
Nitroxinil was invented by May & Baker [2] in the mid 1960s as part of a program into investigation of derivatives of p-hydroxybenzonitrile. In addition to Nitroxynil, the herbicides ioxynil (3,5-diiodo) and bromoxynil (3,5-dibromo) were also invented by the same company. Nitroxynil has a nitro group in addition to a single iodine group.
Nitroxynil is almost insoluble in water. It is usually injected subcutaneously into the animals in the form of the water-soluble ethylglucamine salt. [1] It must not be administered to animals that produce milk for human consumption. [3]
References
- ^ a b "NITROXINIL = NITROXYNIL for veterinary use in CATTLE, SHEEP and GOATS against flukes and roundworms". Retrieved 4 April 2018.
- ^ GB 1104885, May & Baker, "Method for the Treatment of Helminth Infestations", published 18 Dec 1964, issued 6 Mar 1968
- ^ "Committee for Veterinary Medicinal Products, Nitroxinil, Summary Report" (PDF). The European Agency for the Evaluation of Medical Products. June 1998. p. 5. Retrieved 4 April 2018.
External links
 Structure of nitroxinil | |
| Clinical data | |
|---|---|
| Trade names | Fluconix, Dovenix, Trodax |
| Other names | Nitroxynil |
|
Routes of administration | Subcutaneous in the form of an N-Ethylglucamine salt solution |
| ATCvet code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.015.350 |
| Chemical and physical data | |
| Formula | C7H3IN2O3 |
| Molar mass | 290.016 g·mol−1 |
| 3D model ( JSmol) | |
| Melting point | 136–139 °C (277–282 °F) |
| |
| |
Nitroxinil is an anthelmintic, a veterinary medicine against parasitic worms in sheep and cattle. The substance is active against the liver fluke the Fasciola hepatica and to a lesser extent against thread worms in the gastrointestinal tract. [1] Brand names include Fluconix, Dovenix and Trodax. Nitroxynil is also used against strains of the red gum worm ( Haemonchus contortus) that have become resistant to benzimidazoles.[ citation needed]
Nitroxinil was invented by May & Baker [2] in the mid 1960s as part of a program into investigation of derivatives of p-hydroxybenzonitrile. In addition to Nitroxynil, the herbicides ioxynil (3,5-diiodo) and bromoxynil (3,5-dibromo) were also invented by the same company. Nitroxynil has a nitro group in addition to a single iodine group.
Nitroxynil is almost insoluble in water. It is usually injected subcutaneously into the animals in the form of the water-soluble ethylglucamine salt. [1] It must not be administered to animals that produce milk for human consumption. [3]
References
- ^ a b "NITROXINIL = NITROXYNIL for veterinary use in CATTLE, SHEEP and GOATS against flukes and roundworms". Retrieved 4 April 2018.
- ^ GB 1104885, May & Baker, "Method for the Treatment of Helminth Infestations", published 18 Dec 1964, issued 6 Mar 1968
- ^ "Committee for Veterinary Medicinal Products, Nitroxinil, Summary Report" (PDF). The European Agency for the Evaluation of Medical Products. June 1998. p. 5. Retrieved 4 April 2018.