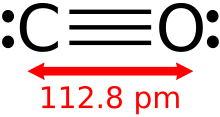
A monoxide is any oxide containing only one atom of oxygen. A well known monoxide is carbon monoxide; see carbon monoxide poisoning.
The prefix mono (Greek for "one") is used in chemical nomenclature. [1] In proper nomenclature, the prefix is not always used in compounds with one oxygen atom. [2] Generally, when the oxygen is bonded to a nonmetal, the prefix mono is used. However, when the oxygen atom bonds to a metal, the prefix is dropped. For instance, in the compound K2O, potassium (K) is a metal and therefore its proper name is potassium oxide, rather than potassium monoxide.
Among monoxides, carbon monoxide and hydrogen monoxide ( water) are both neutral, germanium(II) oxide is distinctly acidic, and both tin(II) oxide and lead(II) oxide are amphoteric.
References
- ^ Foundations of College Chemistry, 13th Edition. Wiley. 2010. p. 110. ISBN 978-0470460610.
- ^ Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005. 2005. pp. 69, 70. ISBN 0-85404-438-8.
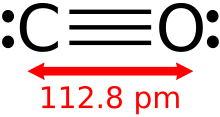
A monoxide is any oxide containing only one atom of oxygen. A well known monoxide is carbon monoxide; see carbon monoxide poisoning.
The prefix mono (Greek for "one") is used in chemical nomenclature. [1] In proper nomenclature, the prefix is not always used in compounds with one oxygen atom. [2] Generally, when the oxygen is bonded to a nonmetal, the prefix mono is used. However, when the oxygen atom bonds to a metal, the prefix is dropped. For instance, in the compound K2O, potassium (K) is a metal and therefore its proper name is potassium oxide, rather than potassium monoxide.
Among monoxides, carbon monoxide and hydrogen monoxide ( water) are both neutral, germanium(II) oxide is distinctly acidic, and both tin(II) oxide and lead(II) oxide are amphoteric.
References
- ^ Foundations of College Chemistry, 13th Edition. Wiley. 2010. p. 110. ISBN 978-0470460610.
- ^ Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005. 2005. pp. 69, 70. ISBN 0-85404-438-8.