| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
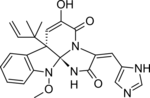
| C23H23N5O4 | |
| Molar mass | 433.468 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Meleagrin and its derivatives such as oxaline are bio-active benzylisoquinoline alkaloids made by various species of Penicillium fungi. [1] It is similar to other fungal alkaloids, such as Roquefortine C, which is made as an intermediate in the same biosynthetic pathway.
It was suggested to have inhibitory activity on fatty acid synthesis for the bacteria Staphylococcus aureus and Streptococcus pneumoniae. [2]
Biosynthesis
The biosynthetic pathway was determined to contain several genes, including a non-ribosomal peptide synthetase. [3] The biosynthesis begins by cyclizing the two amino acids histidine and tryptophan, and is the followed by the addition of an isoprene, and several ring rearrangement steps. [4]
References
- ^ Du, L; Feng, T; Zhao, B; Li, D; Cai, S; Zhu, T; Wang, F; Xiao, X; Gu, Q (April 2010). "Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities". The Journal of Antibiotics. 63 (4): 165–70. doi: 10.1038/ja.2010.11. PMID 20186171. S2CID 12744541.
- ^ Zheng, CJ; Sohn, MJ; Lee, S; Kim, WG (2013). "Meleagrin, a new FabI inhibitor from Penicillium chryosogenum with at least one additional mode of action". PLOS ONE. 8 (11): e78922. Bibcode: 2013PLoSO...878922Z. doi: 10.1371/journal.pone.0078922. PMC 3842914. PMID 24312171.
- ^ Ali, Hazrat; Ries, Marco I.; Nijland, Jeroen G.; Lankhorst, Peter P.; Hankemeier, Thomas; Bovenberg, Roel A. L.; Vreeken, Rob J.; Driessen, Arnold J. M. (12 June 2013). "A Branched Biosynthetic Pathway Is Involved in Production of Roquefortine and Related Compounds in Penicillium chrysogenum". PLOS ONE. 8 (6): e65328. Bibcode: 2013PLoSO...865328A. doi: 10.1371/journal.pone.0065328. PMC 3680398. PMID 23776469.
- ^ García-Estrada, Carlos; Ullán, Ricardo V.; Albillos, Silvia M.; Fernández-Bodega, María Ángeles; Durek, Pawel; von Döhren, Hans; Martín, Juan F. (23 November 2011). "A Single Cluster of Coregulated Genes Encodes the Biosynthesis of the Mycotoxins Roquefortine C and Meleagrin in Penicillium chrysogenum". Chemistry & Biology. 18 (11): 1499–1512. doi: 10.1016/j.chembiol.2011.08.012. PMID 22118684.

| |
| Identifiers | |
|---|---|
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C23H23N5O4 | |
| Molar mass | 433.468 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Meleagrin and its derivatives such as oxaline are bio-active benzylisoquinoline alkaloids made by various species of Penicillium fungi. [1] It is similar to other fungal alkaloids, such as Roquefortine C, which is made as an intermediate in the same biosynthetic pathway.
It was suggested to have inhibitory activity on fatty acid synthesis for the bacteria Staphylococcus aureus and Streptococcus pneumoniae. [2]
Biosynthesis
The biosynthetic pathway was determined to contain several genes, including a non-ribosomal peptide synthetase. [3] The biosynthesis begins by cyclizing the two amino acids histidine and tryptophan, and is the followed by the addition of an isoprene, and several ring rearrangement steps. [4]
References
- ^ Du, L; Feng, T; Zhao, B; Li, D; Cai, S; Zhu, T; Wang, F; Xiao, X; Gu, Q (April 2010). "Alkaloids from a deep ocean sediment-derived fungus Penicillium sp. and their antitumor activities". The Journal of Antibiotics. 63 (4): 165–70. doi: 10.1038/ja.2010.11. PMID 20186171. S2CID 12744541.
- ^ Zheng, CJ; Sohn, MJ; Lee, S; Kim, WG (2013). "Meleagrin, a new FabI inhibitor from Penicillium chryosogenum with at least one additional mode of action". PLOS ONE. 8 (11): e78922. Bibcode: 2013PLoSO...878922Z. doi: 10.1371/journal.pone.0078922. PMC 3842914. PMID 24312171.
- ^ Ali, Hazrat; Ries, Marco I.; Nijland, Jeroen G.; Lankhorst, Peter P.; Hankemeier, Thomas; Bovenberg, Roel A. L.; Vreeken, Rob J.; Driessen, Arnold J. M. (12 June 2013). "A Branched Biosynthetic Pathway Is Involved in Production of Roquefortine and Related Compounds in Penicillium chrysogenum". PLOS ONE. 8 (6): e65328. Bibcode: 2013PLoSO...865328A. doi: 10.1371/journal.pone.0065328. PMC 3680398. PMID 23776469.
- ^ García-Estrada, Carlos; Ullán, Ricardo V.; Albillos, Silvia M.; Fernández-Bodega, María Ángeles; Durek, Pawel; von Döhren, Hans; Martín, Juan F. (23 November 2011). "A Single Cluster of Coregulated Genes Encodes the Biosynthesis of the Mycotoxins Roquefortine C and Meleagrin in Penicillium chrysogenum". Chemistry & Biology. 18 (11): 1499–1512. doi: 10.1016/j.chembiol.2011.08.012. PMID 22118684.