| Arthropod defensin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
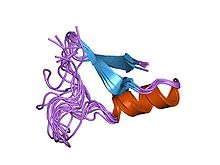 Structure of insect defensin A.
[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Defensin_2 | ||||||||
| Pfam | PF01097 | ||||||||
| InterPro | IPR001542 | ||||||||
| PROSITE | PDOC00356 | ||||||||
| SCOP2 | 1ica / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.47 | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1l4v | ||||||||
| |||||||||
Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. [2] [3] [4] [5] [6] However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. [7] It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence. [7]
Arthropod defensin peptides range in length from 38 to 51 amino acids. There are six conserved cysteines all involved in intrachain disulfide bonds. Studies have shown that the cysteine-bridge disulfide bonds are not required for antimicrobial activity, [8] similar to findings in mammalian defensins. [9] Furthermore, it was also shown that the N-terminal helix region in arthropod or insect defensins is also not required for antimicrobial activity of these peptides. [8]
A schematic representation of peptides from the arthropod defensin family is shown below.
+----------------------------+
| |
xxCxxxxxxxxxxxxxxCxxxCxxxxxxxxxCxxxxxCxCxx
| | | |
+---|---------------+ |
+-----------------+
'C': conserved cysteine involved in a disulfide bond.
Sequence similarities have been reported between the arthropod defensins and mammalian defensins. [10] [2] However it appears that defensins of vertebrates, arthropods, plants, and fungi arose independently. [11] This is supported by 3D structural differences between arthropod defensins and vertebrate beta defensins. [12] However structural similarities exist between these defensins, notably in two structural motifs termed "C6" and "C8". This has prompted a higher "cis-" or "tras-" defensin classification system wherein the structural relationships of the shared motifs is used to delineate defensin similarities. [11]
Defensins of mammals display anti-cancer activities in vitro, [13] and down-regulation of human beta-defensin 1 is associated with increased risk of prostate cancer and clear-cell carcinomas. [14] The first in vivo anti-cancer functions for defensin came from Drosophila studies, which showed that the Drosophila defensin attacks tumor cells, and that flies lacking defensin had greater tumor growth in a cancer disease model. [15] [16]
Overactive immune signalling is also implicated in age-associated neurodegeneration, [17] and overexpression of defensin leads to increased degradation of brain tissue. [18]
- ^ Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F (May 1995). "Refined three-dimensional solution structure of insect defensin A". Structure. 3 (5): 435–48. doi: 10.1016/S0969-2126(01)00177-0. PMID 7663941.
- ^ a b Lambert J, Keppi E, Dimarcq JL, Wicker C, Reichhart JM, Dunbar B, et al. (January 1989). "Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides". Proceedings of the National Academy of Sciences of the United States of America. 86 (1): 262–6. Bibcode: 1989PNAS...86..262L. doi: 10.1073/pnas.86.1.262. PMC 286444. PMID 2911573.
- ^ Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K (July 1990). "A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin". The Journal of Biological Chemistry. 265 (19): 11333–7. doi: 10.1016/S0021-9258(19)38596-5. PMID 2358464.
- ^ Yamada K, Natori S (April 1993). "Purification, sequence and antibacterial activity of two novel sapecin homologues from Sarcophaga embryonic cells: similarity of sapecin B to charybdotoxin". The Biochemical Journal. 291 ( Pt 1) (Pt 1): 275–9. doi: 10.1042/bj2910275. PMC 1132513. PMID 8471044.
- ^ Bulet P, Cociancich S, Dimarcq JL, Lambert J, Reichhart JM, Hoffmann D, et al. (December 1991). "Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family". The Journal of Biological Chemistry. 266 (36): 24520–5. doi: 10.1016/S0021-9258(18)54260-5. PMID 1761552.
- ^ Bulet P, Cociancich S, Reuland M, Sauber F, Bischoff R, Hegy G, et al. (November 1992). "A novel insect defensin mediates the inducible antibacterial activity in larvae of the dragonfly Aeschna cyanea (Paleoptera, Odonata)". European Journal of Biochemistry. 209 (3): 977–84. doi: 10.1111/j.1432-1033.1992.tb17371.x. PMID 1425705.
- ^ a b Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B (February 2019). "Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach". eLife. 8. doi: 10.7554/eLife.44341. PMC 6398976. PMID 30803481.
- ^ a b Varkey J, Singh S, Nagaraj R (November 2006). "Antibacterial activity of linear peptides spanning the carboxy-terminal beta-sheet domain of arthropod defensins". Peptides. 27 (11): 2614–23. doi: 10.1016/j.peptides.2006.06.010. PMID 16914230. S2CID 21104756.
- ^ Varkey J, Nagaraj R (November 2005). "Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines". Antimicrobial Agents and Chemotherapy. 49 (11): 4561–6. doi: 10.1128/AAC.49.11.4561-4566.2005. PMC 1280114. PMID 16251296.
- ^ Rosa RD, Santini A, Fievet J, Bulet P, Destoumieux-Garzón D, Bachère E (2011). "Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas". PLOS ONE. 6 (9): e25594. Bibcode: 2011PLoSO...625594R. doi: 10.1371/journal.pone.0025594. PMC 3182236. PMID 21980497.
- ^ a b Shafee TM, Lay FT, Hulett MD, Anderson MA (September 2016). "The Defensins Consist of Two Independent, Convergent Protein Superfamilies". Molecular Biology and Evolution. 33 (9): 2345–56. doi: 10.1093/molbev/msw106. PMID 27297472.
- ^ Hanzawa H, Shimada I, Kuzuhara T, Komano H, Kohda D, Inagaki F, et al. (September 1990). "1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin". FEBS Letters. 269 (2): 413–20. doi: 10.1016/0014-5793(90)81206-4. PMID 2401368. S2CID 30637946.
- ^ Deslouches B, Di YP (July 2017). "Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications". Oncotarget. 8 (28): 46635–46651. doi: 10.18632/oncotarget.16743. PMC 5542299. PMID 28422728.
- ^ Donald CD, Sun CQ, Lim SD, Macoska J, Cohen C, Amin MB, Young AN, Ganz TA, Marshall FF, Petros JA (April 2003). "Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas". Laboratory Investigation; A Journal of Technical Methods and Pathology. 83 (4): 501–5. doi: 10.1097/01.LAB.0000063929.61760.F6. PMID 12695553.
- ^ Parvy JP, Yu Y, Dostalova A, Kondo S, Kurjan A, Bulet P, Lemaitre B, Vidal M, Cordero JB (July 2019). "The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila". eLife. 8: e45061. doi: 10.7554/eLife.45061. PMC 6667213. PMID 31358113.
- ^ Dawson KP, Abbott GD, Allan J (June 1983). "Acute respiratory infection in childhood: a study of parental prescribing patterns and advice sources". The New Zealand Medical Journal. 96 (734): 481–2. PMID 6602314.
- ^ Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P (April 2017). "NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration". Cell Reports. 19 (4): 836–848. doi: 10.1016/j.celrep.2017.04.007. PMC 5413584. PMID 28445733.
- ^ Cao Y, Chtarbanova S, Petersen AJ, Ganetzky B (May 2013). "Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain". Proceedings of the National Academy of Sciences of the United States of America. 110 (19): E1752–60. Bibcode: 2013PNAS..110E1752C. doi: 10.1073/pnas.1306220110. PMC 3651420. PMID 23613578.
- Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F (May 1995). "Refined three-dimensional solution structure of insect defensin A". Structure. 3 (5): 435–48. doi: 10.1016/S0969-2126(01)00177-0. PMID 7663941.
| Arthropod defensin | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Structure of insect defensin A.
[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Defensin_2 | ||||||||
| Pfam | PF01097 | ||||||||
| InterPro | IPR001542 | ||||||||
| PROSITE | PDOC00356 | ||||||||
| SCOP2 | 1ica / SCOPe / SUPFAM | ||||||||
| TCDB | 1.C.47 | ||||||||
| OPM superfamily | 58 | ||||||||
| OPM protein | 1l4v | ||||||||
| |||||||||
Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. [2] [3] [4] [5] [6] However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. [7] It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence. [7]
Arthropod defensin peptides range in length from 38 to 51 amino acids. There are six conserved cysteines all involved in intrachain disulfide bonds. Studies have shown that the cysteine-bridge disulfide bonds are not required for antimicrobial activity, [8] similar to findings in mammalian defensins. [9] Furthermore, it was also shown that the N-terminal helix region in arthropod or insect defensins is also not required for antimicrobial activity of these peptides. [8]
A schematic representation of peptides from the arthropod defensin family is shown below.
+----------------------------+
| |
xxCxxxxxxxxxxxxxxCxxxCxxxxxxxxxCxxxxxCxCxx
| | | |
+---|---------------+ |
+-----------------+
'C': conserved cysteine involved in a disulfide bond.
Sequence similarities have been reported between the arthropod defensins and mammalian defensins. [10] [2] However it appears that defensins of vertebrates, arthropods, plants, and fungi arose independently. [11] This is supported by 3D structural differences between arthropod defensins and vertebrate beta defensins. [12] However structural similarities exist between these defensins, notably in two structural motifs termed "C6" and "C8". This has prompted a higher "cis-" or "tras-" defensin classification system wherein the structural relationships of the shared motifs is used to delineate defensin similarities. [11]
Defensins of mammals display anti-cancer activities in vitro, [13] and down-regulation of human beta-defensin 1 is associated with increased risk of prostate cancer and clear-cell carcinomas. [14] The first in vivo anti-cancer functions for defensin came from Drosophila studies, which showed that the Drosophila defensin attacks tumor cells, and that flies lacking defensin had greater tumor growth in a cancer disease model. [15] [16]
Overactive immune signalling is also implicated in age-associated neurodegeneration, [17] and overexpression of defensin leads to increased degradation of brain tissue. [18]
- ^ Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F (May 1995). "Refined three-dimensional solution structure of insect defensin A". Structure. 3 (5): 435–48. doi: 10.1016/S0969-2126(01)00177-0. PMID 7663941.
- ^ a b Lambert J, Keppi E, Dimarcq JL, Wicker C, Reichhart JM, Dunbar B, et al. (January 1989). "Insect immunity: isolation from immune blood of the dipteran Phormia terranovae of two insect antibacterial peptides with sequence homology to rabbit lung macrophage bactericidal peptides". Proceedings of the National Academy of Sciences of the United States of America. 86 (1): 262–6. Bibcode: 1989PNAS...86..262L. doi: 10.1073/pnas.86.1.262. PMC 286444. PMID 2911573.
- ^ Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K (July 1990). "A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin". The Journal of Biological Chemistry. 265 (19): 11333–7. doi: 10.1016/S0021-9258(19)38596-5. PMID 2358464.
- ^ Yamada K, Natori S (April 1993). "Purification, sequence and antibacterial activity of two novel sapecin homologues from Sarcophaga embryonic cells: similarity of sapecin B to charybdotoxin". The Biochemical Journal. 291 ( Pt 1) (Pt 1): 275–9. doi: 10.1042/bj2910275. PMC 1132513. PMID 8471044.
- ^ Bulet P, Cociancich S, Dimarcq JL, Lambert J, Reichhart JM, Hoffmann D, et al. (December 1991). "Insect immunity. Isolation from a coleopteran insect of a novel inducible antibacterial peptide and of new members of the insect defensin family". The Journal of Biological Chemistry. 266 (36): 24520–5. doi: 10.1016/S0021-9258(18)54260-5. PMID 1761552.
- ^ Bulet P, Cociancich S, Reuland M, Sauber F, Bischoff R, Hegy G, et al. (November 1992). "A novel insect defensin mediates the inducible antibacterial activity in larvae of the dragonfly Aeschna cyanea (Paleoptera, Odonata)". European Journal of Biochemistry. 209 (3): 977–84. doi: 10.1111/j.1432-1033.1992.tb17371.x. PMID 1425705.
- ^ a b Hanson MA, Dostálová A, Ceroni C, Poidevin M, Kondo S, Lemaitre B (February 2019). "Synergy and remarkable specificity of antimicrobial peptides in vivo using a systematic knockout approach". eLife. 8. doi: 10.7554/eLife.44341. PMC 6398976. PMID 30803481.
- ^ a b Varkey J, Singh S, Nagaraj R (November 2006). "Antibacterial activity of linear peptides spanning the carboxy-terminal beta-sheet domain of arthropod defensins". Peptides. 27 (11): 2614–23. doi: 10.1016/j.peptides.2006.06.010. PMID 16914230. S2CID 21104756.
- ^ Varkey J, Nagaraj R (November 2005). "Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines". Antimicrobial Agents and Chemotherapy. 49 (11): 4561–6. doi: 10.1128/AAC.49.11.4561-4566.2005. PMC 1280114. PMID 16251296.
- ^ Rosa RD, Santini A, Fievet J, Bulet P, Destoumieux-Garzón D, Bachère E (2011). "Big defensins, a diverse family of antimicrobial peptides that follows different patterns of expression in hemocytes of the oyster Crassostrea gigas". PLOS ONE. 6 (9): e25594. Bibcode: 2011PLoSO...625594R. doi: 10.1371/journal.pone.0025594. PMC 3182236. PMID 21980497.
- ^ a b Shafee TM, Lay FT, Hulett MD, Anderson MA (September 2016). "The Defensins Consist of Two Independent, Convergent Protein Superfamilies". Molecular Biology and Evolution. 33 (9): 2345–56. doi: 10.1093/molbev/msw106. PMID 27297472.
- ^ Hanzawa H, Shimada I, Kuzuhara T, Komano H, Kohda D, Inagaki F, et al. (September 1990). "1H nuclear magnetic resonance study of the solution conformation of an antibacterial protein, sapecin". FEBS Letters. 269 (2): 413–20. doi: 10.1016/0014-5793(90)81206-4. PMID 2401368. S2CID 30637946.
- ^ Deslouches B, Di YP (July 2017). "Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications". Oncotarget. 8 (28): 46635–46651. doi: 10.18632/oncotarget.16743. PMC 5542299. PMID 28422728.
- ^ Donald CD, Sun CQ, Lim SD, Macoska J, Cohen C, Amin MB, Young AN, Ganz TA, Marshall FF, Petros JA (April 2003). "Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas". Laboratory Investigation; A Journal of Technical Methods and Pathology. 83 (4): 501–5. doi: 10.1097/01.LAB.0000063929.61760.F6. PMID 12695553.
- ^ Parvy JP, Yu Y, Dostalova A, Kondo S, Kurjan A, Bulet P, Lemaitre B, Vidal M, Cordero JB (July 2019). "The antimicrobial peptide defensin cooperates with tumour necrosis factor to drive tumour cell death in Drosophila". eLife. 8: e45061. doi: 10.7554/eLife.45061. PMC 6667213. PMID 31358113.
- ^ Dawson KP, Abbott GD, Allan J (June 1983). "Acute respiratory infection in childhood: a study of parental prescribing patterns and advice sources". The New Zealand Medical Journal. 96 (734): 481–2. PMID 6602314.
- ^ Kounatidis I, Chtarbanova S, Cao Y, Hayne M, Jayanth D, Ganetzky B, Ligoxygakis P (April 2017). "NF-κB Immunity in the Brain Determines Fly Lifespan in Healthy Aging and Age-Related Neurodegeneration". Cell Reports. 19 (4): 836–848. doi: 10.1016/j.celrep.2017.04.007. PMC 5413584. PMID 28445733.
- ^ Cao Y, Chtarbanova S, Petersen AJ, Ganetzky B (May 2013). "Dnr1 mutations cause neurodegeneration in Drosophila by activating the innate immune response in the brain". Proceedings of the National Academy of Sciences of the United States of America. 110 (19): E1752–60. Bibcode: 2013PNAS..110E1752C. doi: 10.1073/pnas.1306220110. PMC 3651420. PMID 23613578.
- Cornet B, Bonmatin JM, Hetru C, Hoffmann JA, Ptak M, Vovelle F (May 1995). "Refined three-dimensional solution structure of insect defensin A". Structure. 3 (5): 435–48. doi: 10.1016/S0969-2126(01)00177-0. PMID 7663941.