 | |
| Clinical data | |
|---|---|
| Trade names | Careram; Kolbet |
| Other names | T-614 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.236.037 |
| Chemical and physical data | |
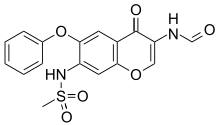
| Formula | C17H14N2O6S |
| Molar mass | 374.37 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Iguratimod is an anti-inflammatory small molecule drug used for the treatment of rheumatoid arthritis, together with methotrexate in Japan and China. [1] As of 2015 the biological target was not known, but it prevents NF-κB activation and subsequently selectively inhibits COX-2 and several inflammatory cytokines. [1]
Adverse effects include elevated transaminases, nausea, vomiting, stomach pain; rashes, and itchiness. [1]
It is a derivative of 7-methanesulfonylamino-6-phenoxychromones and is a chromone with two amide groups; it was first published in 2000. [1] [2] It was submitted for regulatory approval in Japan in 2003; the application was withdrawn in 2009, and it was resubmitted with additional data in 2011 and approved for marketing in Japan in 2012. [1] Eisai and Toyama Chemical market it in Japan. [3] Approval was obtained in China in 2011 by Simcere, independently of the Japanese originators. [1] [4]
During discovery and development it was called T-614 and it is marketed under the names Careram and Kolbet. [5]
References
- ^ a b c d e f Tanaka K, Yamaguchi T, Hara M (May 2015). "Iguratimod for the treatment of rheumatoid arthritis in Japan". Expert Review of Clinical Immunology. 11 (5): 565–73. doi: 10.1586/1744666X.2015.1027151. PMID 25797025. S2CID 25134255.
- ^ Inaba T, Tanaka K, Takeno R, Nagaki H, Yoshida C, Takano S (January 2000). "Synthesis and antiinflammatory activity of 7-methanesulfonylamino-6-phenoxychromones. Antiarthritic effect of the 3-formylamino compound (T-614) in chronic inflammatory disease models". Chemical & Pharmaceutical Bulletin. 48 (1): 131–9. doi: 10.1248/cpb.48.131. PMID 10705489.
- ^ Bronson J, Dhar M, Ewing W, Lonberg N (2012). "Chapter Thirty-One – To Market, To Market—2011". Annual Reports in Medicinal Chemistry. 47: 499–569. doi: 10.1016/B978-0-12-396492-2.00031-X.
- ^ "Iguratimod - Simcere". AdisInsight. Retrieved 27 May 2018.
- ^ "Iguratimod - Toyama Chemical". AdisInsight. Retrieved 27 May 2018.
 | |
| Clinical data | |
|---|---|
| Trade names | Careram; Kolbet |
| Other names | T-614 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| ECHA InfoCard | 100.236.037 |
| Chemical and physical data | |
| Formula | C17H14N2O6S |
| Molar mass | 374.37 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Iguratimod is an anti-inflammatory small molecule drug used for the treatment of rheumatoid arthritis, together with methotrexate in Japan and China. [1] As of 2015 the biological target was not known, but it prevents NF-κB activation and subsequently selectively inhibits COX-2 and several inflammatory cytokines. [1]
Adverse effects include elevated transaminases, nausea, vomiting, stomach pain; rashes, and itchiness. [1]
It is a derivative of 7-methanesulfonylamino-6-phenoxychromones and is a chromone with two amide groups; it was first published in 2000. [1] [2] It was submitted for regulatory approval in Japan in 2003; the application was withdrawn in 2009, and it was resubmitted with additional data in 2011 and approved for marketing in Japan in 2012. [1] Eisai and Toyama Chemical market it in Japan. [3] Approval was obtained in China in 2011 by Simcere, independently of the Japanese originators. [1] [4]
During discovery and development it was called T-614 and it is marketed under the names Careram and Kolbet. [5]
References
- ^ a b c d e f Tanaka K, Yamaguchi T, Hara M (May 2015). "Iguratimod for the treatment of rheumatoid arthritis in Japan". Expert Review of Clinical Immunology. 11 (5): 565–73. doi: 10.1586/1744666X.2015.1027151. PMID 25797025. S2CID 25134255.
- ^ Inaba T, Tanaka K, Takeno R, Nagaki H, Yoshida C, Takano S (January 2000). "Synthesis and antiinflammatory activity of 7-methanesulfonylamino-6-phenoxychromones. Antiarthritic effect of the 3-formylamino compound (T-614) in chronic inflammatory disease models". Chemical & Pharmaceutical Bulletin. 48 (1): 131–9. doi: 10.1248/cpb.48.131. PMID 10705489.
- ^ Bronson J, Dhar M, Ewing W, Lonberg N (2012). "Chapter Thirty-One – To Market, To Market—2011". Annual Reports in Medicinal Chemistry. 47: 499–569. doi: 10.1016/B978-0-12-396492-2.00031-X.
- ^ "Iguratimod - Simcere". AdisInsight. Retrieved 27 May 2018.
- ^ "Iguratimod - Toyama Chemical". AdisInsight. Retrieved 27 May 2018.