| |
| Names | |
|---|---|
|
Preferred IUPAC name
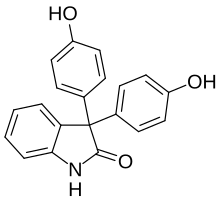
3,3-Bis(4-hydroxyphenyl)-1,3-dihydro-2H-indol-2-one | |
| Other names | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.299 |
| EC Number |
|
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H15NO3 | |
| Molar mass | 317.344 g·mol−1 |
| log P | 1.398 |
| Acidity (pKa) | 9.423 |
| Basicity (pKb) | 4.574 |
| Pharmacology | |
| A06AB01 ( WHO) | |
| Oral, rectal | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Oxyphenisatine (or oxyphenisatin) is a laxative. [3] It is closely related to bisacodyl, sodium picosulfate, and phenolphthalein. Long-term use is associated with liver damage, [4] and as a result, it was withdrawn in most countries in the early 1970s. The acetate derivative oxyphenisatine acetate was also once used as a laxative.
Natural chemical compounds similar to oxyphenisatine may be present in prunes, [5] but a recent review of the relevant scientific literature suggests that the laxative effect of prunes is due to other constituents including phenolic compounds (mainly neochlorogenic acids and chlorogenic acids) and sorbitol. [6] Oxyphenisatin has cathartic properties.
Synthesis
The ketone group of isatin (1) is nonenolizable and has interesting properties. In strong acid it becomes protonated, and the oxygen can be replaced by electron rich moieties.

In 1885, it was reported that condensation of isatin with phenol 2 leads to oxyphenisatin (3), which can then also be acetylated to (4).
Derivatives
- Phenisatin (Triacetyldiphenolisatin, Laxagetten, Unilax, Trisatin)
- Nicoxyphenisatin
- Cinnoxyphenisatin
- Cofisatine [54063-34-2]
References
- ^ a b c SciFinder Scholar, version 2004.2; Chemical Abstracts Service, Registry Number 125-13-3, accessed September 1, 2011
- ^ 21 CFR 216.24
- ^ Farack, U. M.; Nell, G. (1984). "Mechanism of Action of Diphenolic Laxatives: The Role of Adenylate Cyclase and Mucosal Permeability". Digestion. 30 (3): 191–194. doi: 10.1159/000199105. PMID 6548720.
- ^ Kotha, P.; Rake, M. O.; Willatt, D. (1980). "Liver Damage Induced by Oxyphenisatin". British Medical Journal. 281 (6254): 1530. doi: 10.1136/bmj.281.6254.1530. PMC 1714947. PMID 6893676.
- ^ Baum, H. M.; Sanders, R. G.; Straub, G. J. (1951). "The Occurrence of a Diphenyl Isatin in California Prunes". Journal of the American Pharmaceutical Association. 40 (7): 348–349. doi: 10.1002/jps.3030400713. PMID 14850362.
- ^ Stacewicz-Sapuntzakis, M.; Bowen, P. E.; Hussain, E. A.; Damayanti-Wood, B. I.; Farnsworth, N. R. (2001). "Chemical Composition and Potential Health Effects of Prunes: A Functional Food?". Critical Reviews in Food Science and Nutrition. 41 (4): 251–286. doi: 10.1080/20014091091814. PMID 11401245. S2CID 31159565.
- ^ Baeyer, A.; Lazarus, M. J. (1885). "Ueber Condensationsproducte des Isatins". Berichte der Deutschen Chemischen Gesellschaft. 18 (2): 2637. doi: 10.1002/cber.188501802170.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
3,3-Bis(4-hydroxyphenyl)-1,3-dihydro-2H-indol-2-one | |
| Other names | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.004.299 |
| EC Number |
|
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H15NO3 | |
| Molar mass | 317.344 g·mol−1 |
| log P | 1.398 |
| Acidity (pKa) | 9.423 |
| Basicity (pKb) | 4.574 |
| Pharmacology | |
| A06AB01 ( WHO) | |
| Oral, rectal | |
| Legal status |
|
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Oxyphenisatine (or oxyphenisatin) is a laxative. [3] It is closely related to bisacodyl, sodium picosulfate, and phenolphthalein. Long-term use is associated with liver damage, [4] and as a result, it was withdrawn in most countries in the early 1970s. The acetate derivative oxyphenisatine acetate was also once used as a laxative.
Natural chemical compounds similar to oxyphenisatine may be present in prunes, [5] but a recent review of the relevant scientific literature suggests that the laxative effect of prunes is due to other constituents including phenolic compounds (mainly neochlorogenic acids and chlorogenic acids) and sorbitol. [6] Oxyphenisatin has cathartic properties.
Synthesis
The ketone group of isatin (1) is nonenolizable and has interesting properties. In strong acid it becomes protonated, and the oxygen can be replaced by electron rich moieties.

In 1885, it was reported that condensation of isatin with phenol 2 leads to oxyphenisatin (3), which can then also be acetylated to (4).
Derivatives
- Phenisatin (Triacetyldiphenolisatin, Laxagetten, Unilax, Trisatin)
- Nicoxyphenisatin
- Cinnoxyphenisatin
- Cofisatine [54063-34-2]
References
- ^ a b c SciFinder Scholar, version 2004.2; Chemical Abstracts Service, Registry Number 125-13-3, accessed September 1, 2011
- ^ 21 CFR 216.24
- ^ Farack, U. M.; Nell, G. (1984). "Mechanism of Action of Diphenolic Laxatives: The Role of Adenylate Cyclase and Mucosal Permeability". Digestion. 30 (3): 191–194. doi: 10.1159/000199105. PMID 6548720.
- ^ Kotha, P.; Rake, M. O.; Willatt, D. (1980). "Liver Damage Induced by Oxyphenisatin". British Medical Journal. 281 (6254): 1530. doi: 10.1136/bmj.281.6254.1530. PMC 1714947. PMID 6893676.
- ^ Baum, H. M.; Sanders, R. G.; Straub, G. J. (1951). "The Occurrence of a Diphenyl Isatin in California Prunes". Journal of the American Pharmaceutical Association. 40 (7): 348–349. doi: 10.1002/jps.3030400713. PMID 14850362.
- ^ Stacewicz-Sapuntzakis, M.; Bowen, P. E.; Hussain, E. A.; Damayanti-Wood, B. I.; Farnsworth, N. R. (2001). "Chemical Composition and Potential Health Effects of Prunes: A Functional Food?". Critical Reviews in Food Science and Nutrition. 41 (4): 251–286. doi: 10.1080/20014091091814. PMID 11401245. S2CID 31159565.
- ^ Baeyer, A.; Lazarus, M. J. (1885). "Ueber Condensationsproducte des Isatins". Berichte der Deutschen Chemischen Gesellschaft. 18 (2): 2637. doi: 10.1002/cber.188501802170.