This article may need to be rewritten to comply with Wikipedia's
quality standards. (March 2020) |
| |
| Names | |
|---|---|
|
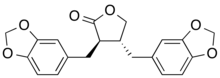
Preferred IUPAC name
(3R,4R)-3,4-Bis[(2H-1,3-benzodioxol-5-yl)methyl]oxolan-2-one | |
| Other names
(3R,4R)-3,4-Bis(1,3-benzodioxol-5-ylmethyl)dihydro-2(3H)-furanone
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H18O6 | |
| Molar mass | 354.358 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Hinokinin is a dibenzylbutyrolactone lignan, derived from various species of plants. It is a potential antichagonistic agent. [1] In vitro, it has been shown to have potential neuroprotective effects [2] as well as anti-inflammatory, anti-tumor, antiviral and antifungal properties. [3]
Hinokinin was isolated for the first time by Yoshiki and Ishiguro in 1933 from hinoki wood. [4]
Chemical properties
Hinokinin is a colourless crystalline compound.[ citation needed]
It can be isolated from various species of Chamaecyparis, Zanthoxylum, Phyllanthus, Aristolochia, Piper, Virola, Linum and Bursera. It is also synthesised from pinoresinol. [1]
Biological effects
Cytotoxic actions
Hinokinin has shown to induce apoptosis and promote antitumor actions on various cancer cell lines in vitro. [5]
Anti-inflammatory actions
Hinokinin has been shown to inhibit the generation of superoxide molecules by neutrophils and also decreases elastase secretion from neutrophils. [6] It has also shown to reduce LPS induced nitric oxide production from macrophages.The anti-inflammatory property of hinokinin is mediated by the NF-kB signalling mechanism. [7]
Anti-parasitic actions
Hinokinin has been shown to be an antitrypanosomal agent. Its use as a treatment for trypanosomiasis is still being researched. [8]
Anti-viral actions
It has shown significant antiviral activity against human hepatitis B virus, HIV and SARS-CoV. [9]
See also
References
- ^ a b Marcotullio, Maria; Pelosi, Azzurra; Curini, Massimo (2014-09-17). "Hinokinin, an Emerging Bioactive Lignan". Molecules. 19 (9): 14862–14878. doi: 10.3390/molecules190914862. ISSN 1420-3049. PMC 6271885. PMID 25232707.
- ^ Timple, Julie Marie V.; Magalhães, Lizandra Guidi; Souza Rezende, Karen Cristina; Pereira, Ana Carolina; Cunha, Wilson Roberto; Andrade e Silva, Márcio Luis; Mortensen, Ole Valente; Fontana, Andréia C. K. (2013-10-10). "The Lignan (−)-Hinokinin Displays Modulatory Effects on Human Monoamine and GABA Transporter Activities". Journal of Natural Products. 76 (10): 1889–1895. doi: 10.1021/np400452n. ISSN 0163-3864. PMID 24112084.
- ^ Zhou, Qi-Long; Wang, Hui-Jing; Tang, Pei; Song, Hao; Qin, Yong (October 2015). "Total Synthesis of Lignan Lactone (–)-Hinokinin". Natural Products and Bioprospecting. 5 (5): 255–261. doi: 10.1007/s13659-015-0073-3. ISSN 2192-2195. PMC 4607678. PMID 26458924.
- ^ Yoshiki, Y.; Ishiguro, T. (1933). "Ueber die kristallisierten Bestandteile des Hinokiöls". Yakugaku Zasshi. 53 (2): 73–151. doi: 10.1248/yakushi1881.53.2_73. ISSN 0031-6903.
- ^ Cao, Xue-li; Xu, Jing; Bai, Ge; Zhang, Hong; Liu, Yan; Xiang, Jun-feng; Tang, Ya-lin (June 2013). "Isolation of anti-tumor compounds from the stem bark of Zanthoxylum ailanthoides Sieb. & Zucc. by silica gel column and counter-current chromatography". Journal of Chromatography B. 929: 6–10. doi: 10.1016/j.jchromb.2013.04.006. ISSN 1570-0232. PMID 23660246.
- ^ Chen, JJ; Chung, CY; Hwang, TL; Chen, JF (July 2009). "Amides and Benzenoids from Zanthoxylum ailanthoides with Inhibitory Activity on Superoxide Generation and Elastase Release by Neutrophils". Planta Medica. 75 (9). doi: 10.1055/s-0029-1234991. ISSN 0032-0943.
- ^ Desai, Dattatraya C.; Jacob, Jeenu; Almeida, Asha; Kshirsagar, Rajendra; Manju, S.L. (2014-05-23). "Isolation, structural elucidation and anti-inflammatory activity of astragalin, ( − )hinokinin, aristolactam I and aristolochic acids (I & II) fromAristolochia indica". Natural Product Research. 28 (17): 1413–1417. doi: 10.1080/14786419.2014.905563. ISSN 1478-6419. PMID 24854204. S2CID 20632496.
- ^ Saraiva, Juliana; Lira, Ana Amélia Moreira; Esperandim, Viviane Rodrigues; da Silva Ferreira, Daniele; Ferraudo, Antônio Sérgio; Bastos, Jairo Kenupp; e Silva, Márcio Luís Andrade; de Gaitani, Cristiane Masetto; de Albuquerque, Sérgio; Marchetti, Juliana Maldonado (2010-01-28). "(−)−Hinokinin-loaded poly(D,L-lactide-co-glycolide) microparticles for Chagas disease". Parasitology Research. 106 (3): 703–708. doi: 10.1007/s00436-010-1725-1. ISSN 0932-0113. PMID 20107838. S2CID 31990739.
- ^ Wen, Chih-Chun; Kuo, Yueh-Hsiung; Jan, Jia-Tsrong; Liang, Po-Huang; Wang, Sheng-Yang; Liu, Hong-Gi; Lee, Ching-Kuo; Chang, Shang-Tzen; Kuo, Chih-Jung; Lee, Shoei-Sheng; Hou, Chia-Chung (August 2007). "Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus" (PDF). Journal of Medicinal Chemistry. 50 (17): 4087–4095. doi: 10.1021/jm070295s. ISSN 0022-2623. PMID 17663539.
This article may need to be rewritten to comply with Wikipedia's
quality standards. (March 2020) |

| |
| Names | |
|---|---|
|
Preferred IUPAC name
(3R,4R)-3,4-Bis[(2H-1,3-benzodioxol-5-yl)methyl]oxolan-2-one | |
| Other names
(3R,4R)-3,4-Bis(1,3-benzodioxol-5-ylmethyl)dihydro-2(3H)-furanone
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H18O6 | |
| Molar mass | 354.358 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Hinokinin is a dibenzylbutyrolactone lignan, derived from various species of plants. It is a potential antichagonistic agent. [1] In vitro, it has been shown to have potential neuroprotective effects [2] as well as anti-inflammatory, anti-tumor, antiviral and antifungal properties. [3]
Hinokinin was isolated for the first time by Yoshiki and Ishiguro in 1933 from hinoki wood. [4]
Chemical properties
Hinokinin is a colourless crystalline compound.[ citation needed]
It can be isolated from various species of Chamaecyparis, Zanthoxylum, Phyllanthus, Aristolochia, Piper, Virola, Linum and Bursera. It is also synthesised from pinoresinol. [1]
Biological effects
Cytotoxic actions
Hinokinin has shown to induce apoptosis and promote antitumor actions on various cancer cell lines in vitro. [5]
Anti-inflammatory actions
Hinokinin has been shown to inhibit the generation of superoxide molecules by neutrophils and also decreases elastase secretion from neutrophils. [6] It has also shown to reduce LPS induced nitric oxide production from macrophages.The anti-inflammatory property of hinokinin is mediated by the NF-kB signalling mechanism. [7]
Anti-parasitic actions
Hinokinin has been shown to be an antitrypanosomal agent. Its use as a treatment for trypanosomiasis is still being researched. [8]
Anti-viral actions
It has shown significant antiviral activity against human hepatitis B virus, HIV and SARS-CoV. [9]
See also
References
- ^ a b Marcotullio, Maria; Pelosi, Azzurra; Curini, Massimo (2014-09-17). "Hinokinin, an Emerging Bioactive Lignan". Molecules. 19 (9): 14862–14878. doi: 10.3390/molecules190914862. ISSN 1420-3049. PMC 6271885. PMID 25232707.
- ^ Timple, Julie Marie V.; Magalhães, Lizandra Guidi; Souza Rezende, Karen Cristina; Pereira, Ana Carolina; Cunha, Wilson Roberto; Andrade e Silva, Márcio Luis; Mortensen, Ole Valente; Fontana, Andréia C. K. (2013-10-10). "The Lignan (−)-Hinokinin Displays Modulatory Effects on Human Monoamine and GABA Transporter Activities". Journal of Natural Products. 76 (10): 1889–1895. doi: 10.1021/np400452n. ISSN 0163-3864. PMID 24112084.
- ^ Zhou, Qi-Long; Wang, Hui-Jing; Tang, Pei; Song, Hao; Qin, Yong (October 2015). "Total Synthesis of Lignan Lactone (–)-Hinokinin". Natural Products and Bioprospecting. 5 (5): 255–261. doi: 10.1007/s13659-015-0073-3. ISSN 2192-2195. PMC 4607678. PMID 26458924.
- ^ Yoshiki, Y.; Ishiguro, T. (1933). "Ueber die kristallisierten Bestandteile des Hinokiöls". Yakugaku Zasshi. 53 (2): 73–151. doi: 10.1248/yakushi1881.53.2_73. ISSN 0031-6903.
- ^ Cao, Xue-li; Xu, Jing; Bai, Ge; Zhang, Hong; Liu, Yan; Xiang, Jun-feng; Tang, Ya-lin (June 2013). "Isolation of anti-tumor compounds from the stem bark of Zanthoxylum ailanthoides Sieb. & Zucc. by silica gel column and counter-current chromatography". Journal of Chromatography B. 929: 6–10. doi: 10.1016/j.jchromb.2013.04.006. ISSN 1570-0232. PMID 23660246.
- ^ Chen, JJ; Chung, CY; Hwang, TL; Chen, JF (July 2009). "Amides and Benzenoids from Zanthoxylum ailanthoides with Inhibitory Activity on Superoxide Generation and Elastase Release by Neutrophils". Planta Medica. 75 (9). doi: 10.1055/s-0029-1234991. ISSN 0032-0943.
- ^ Desai, Dattatraya C.; Jacob, Jeenu; Almeida, Asha; Kshirsagar, Rajendra; Manju, S.L. (2014-05-23). "Isolation, structural elucidation and anti-inflammatory activity of astragalin, ( − )hinokinin, aristolactam I and aristolochic acids (I & II) fromAristolochia indica". Natural Product Research. 28 (17): 1413–1417. doi: 10.1080/14786419.2014.905563. ISSN 1478-6419. PMID 24854204. S2CID 20632496.
- ^ Saraiva, Juliana; Lira, Ana Amélia Moreira; Esperandim, Viviane Rodrigues; da Silva Ferreira, Daniele; Ferraudo, Antônio Sérgio; Bastos, Jairo Kenupp; e Silva, Márcio Luís Andrade; de Gaitani, Cristiane Masetto; de Albuquerque, Sérgio; Marchetti, Juliana Maldonado (2010-01-28). "(−)−Hinokinin-loaded poly(D,L-lactide-co-glycolide) microparticles for Chagas disease". Parasitology Research. 106 (3): 703–708. doi: 10.1007/s00436-010-1725-1. ISSN 0932-0113. PMID 20107838. S2CID 31990739.
- ^ Wen, Chih-Chun; Kuo, Yueh-Hsiung; Jan, Jia-Tsrong; Liang, Po-Huang; Wang, Sheng-Yang; Liu, Hong-Gi; Lee, Ching-Kuo; Chang, Shang-Tzen; Kuo, Chih-Jung; Lee, Shoei-Sheng; Hou, Chia-Chung (August 2007). "Specific Plant Terpenoids and Lignoids Possess Potent Antiviral Activities against Severe Acute Respiratory Syndrome Coronavirus" (PDF). Journal of Medicinal Chemistry. 50 (17): 4087–4095. doi: 10.1021/jm070295s. ISSN 0022-2623. PMID 17663539.