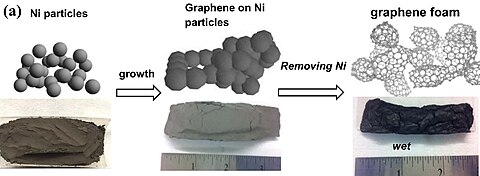

Graphene foam is a solid, open-cell foam made of single-layer sheets of graphene. [1] [2] It is a candidate substrate for the electrode of lithium-ion batteries.
The foam can be manufactured using vapor deposition to coat a metal foam, a three-dimensional mesh of metal filaments. The metal is then removed. [1]
A physically flexible battery was created using the foam for electrodes. The anode was made by coating the foam with a lithium-titanium compound (Li
4Ti
5O
12) and the
cathode by coating the foam with LiFePO
4. Both electrodes were lightweight and their large surface area provided high
energy density of 110 Wh/kg, comparable to commercial batteries.
[1]
Power density was much greater than a typical battery. At a rate that completely discharged the material in 18 seconds, power delivered was 80 percent of what it produced during an hour-long discharge. Performance remained stable through 500 charge/discharge cycles. [1]
In 2017 researchers used carbon nanotubes to reinforce a foam. The latter material supports 3,000 times its own weight and can return to its original shape when unweighted. Nanotubes, a powdered nickel catalyst and sugar were mixed. Dried pellets of the substance were then compressed in a steel die in the shape of a screw. The nickel was removed, leaving a screw-shaped piece of foam. The nanotubes' outer layers split and bonded with the graphene. [3]
- ^ a b c d Li, Na; Chen, Zongping; Ren, Wencai; Li, Feng; Cheng, Hui-Ming (23 October 2012). "Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates". Proceedings of the National Academy of Sciences. 109 (43): 17360–17365. Bibcode: 2012PNAS..10917360L. doi: 10.1073/pnas.1210072109. PMC 3491507. PMID 23045691. + Supplement.
- ^ Timmer, John (2012). "The fast and the flexible: Graphene foam batteries charge quickly". Ars Technica.
- ^ Irving, Michael (2017-02-13). ""Rebar graphene" foam supports 3,000 times its own weight". newatlas.com. Retrieved 2017-02-15.
- Paronyan, Tereza M.; Thapa, Arjun Kumar; Sherehiy, Andriy; Jasinski, Jacek B.; Jangam, John Samuel Dilip (6 January 2017). "Incommensurate Graphene Foam as a High Capacity Lithium Intercalation Anode". Scientific Reports. 7 (1): 39944. Bibcode: 2017NatSR...739944P. doi: 10.1038/srep39944. PMC 5216342. PMID 28059110.


Graphene foam is a solid, open-cell foam made of single-layer sheets of graphene. [1] [2] It is a candidate substrate for the electrode of lithium-ion batteries.
The foam can be manufactured using vapor deposition to coat a metal foam, a three-dimensional mesh of metal filaments. The metal is then removed. [1]
A physically flexible battery was created using the foam for electrodes. The anode was made by coating the foam with a lithium-titanium compound (Li
4Ti
5O
12) and the
cathode by coating the foam with LiFePO
4. Both electrodes were lightweight and their large surface area provided high
energy density of 110 Wh/kg, comparable to commercial batteries.
[1]
Power density was much greater than a typical battery. At a rate that completely discharged the material in 18 seconds, power delivered was 80 percent of what it produced during an hour-long discharge. Performance remained stable through 500 charge/discharge cycles. [1]
In 2017 researchers used carbon nanotubes to reinforce a foam. The latter material supports 3,000 times its own weight and can return to its original shape when unweighted. Nanotubes, a powdered nickel catalyst and sugar were mixed. Dried pellets of the substance were then compressed in a steel die in the shape of a screw. The nickel was removed, leaving a screw-shaped piece of foam. The nanotubes' outer layers split and bonded with the graphene. [3]
- ^ a b c d Li, Na; Chen, Zongping; Ren, Wencai; Li, Feng; Cheng, Hui-Ming (23 October 2012). "Flexible graphene-based lithium ion batteries with ultrafast charge and discharge rates". Proceedings of the National Academy of Sciences. 109 (43): 17360–17365. Bibcode: 2012PNAS..10917360L. doi: 10.1073/pnas.1210072109. PMC 3491507. PMID 23045691. + Supplement.
- ^ Timmer, John (2012). "The fast and the flexible: Graphene foam batteries charge quickly". Ars Technica.
- ^ Irving, Michael (2017-02-13). ""Rebar graphene" foam supports 3,000 times its own weight". newatlas.com. Retrieved 2017-02-15.
- Paronyan, Tereza M.; Thapa, Arjun Kumar; Sherehiy, Andriy; Jasinski, Jacek B.; Jangam, John Samuel Dilip (6 January 2017). "Incommensurate Graphene Foam as a High Capacity Lithium Intercalation Anode". Scientific Reports. 7 (1): 39944. Bibcode: 2017NatSR...739944P. doi: 10.1038/srep39944. PMC 5216342. PMID 28059110.