| SPTAN1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SPTAN1, EIEE5, NEAS, SPTA2, spectrin alpha, non-erythrocytic 1, DEE5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 182810; MGI: 98386; HomoloGene: 2353; GeneCards: SPTAN1; OMA: SPTAN1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
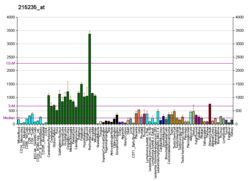
Alpha II-spectrin, also known as Spectrin alpha chain, brain is a protein that in humans is encoded by the SPTAN1 gene. [5] [6] [7] Alpha II-spectrin is expressed in a variety of tissues, and is highly expressed in cardiac muscle at Z-disc structures, costameres and at the sarcolemma membrane. Mutations in alpha II-spectrin have been associated with early infantile epileptic encephalopathy-5, and alpha II-spectrin may be a valuable biomarker for Guillain–Barré syndrome and infantile congenital heart disease.
Structure
Alternate splicing of alpha II-spectrin has been documented and results in multiple transcript variants; specifically, cardiomyocytes have four identified alpha II-spectrin splice variants. [8] [9] As opposed to alpha I-spectrin that is principally found in erythrocytes, [10] alpha II-spectrin is expressed in most tissues. In cardiac tissue, alpha II-spectrin is found in myocytes at Z-discs, costameres, and the sarcolemma membrane, [11] [12] [13] and in cardiac fibroblasts along the surface of the cytoskeletal network. [14] Alpha II-spectrin most commonly exists in a heterodimer with alpha II and beta II spectrin subunits; and dimers typically self-associate and heterotetramerize. [5] [15] [16]
Function
The spectrins are a family of widely distributed cytoskeletal proteins which are involved in actin crosslinking, cell adhesion, intercellular communication and cell cycle regulation. [17] [18] [19] Though a role in cardiac muscle is not well understood, it is likely that alpha II-spectrin is involved in organizing sub- sarcolemmal domains and stabilizing sarcolemmal membranes against the stresses associated with continuous cardiac contraction. [16] Functional diversity of alpha II-spectrin is manifest through its four splice variants. First, a cardiac-specific, 21 amino acid sequence insert in the 21st spectrin repeat, termed alpha II-cardi+, was identified as an insert that modulates affinity of alpha II-spectrin for binding beta-spectrins and regulates myocyte growth and differentiation. [8] Secondly, another insert of 20 amino acids in the 10th spectrin repeat, termed SH3i+, contains protein kinase A and protein kinase C phosphorylation sites and modulates Ca2+-dependent cleavage of spectrin and protein-protein interaction properties. [20] Thirdly, an insert of five amino acids in the fifteenth spectrin motif bears a highly antigenic epitope resembling an ankyrin-like p53 binding protein binding site. [8] [21] Fourthly, a six amino acid insert in the twenty-first spectrin motif with unknown function has been reported. [11] [22]
Alpha II-spectrin gene expression has been shown to be upregulated in cardiac fibroblasts in response to Angiotensin II-induced cardiac remodeling. [23]
In animal models of disease and injury, alpha II-spectrin has been implicated in diverse functions. In a canine model of hypothermic circulatory arrest, alpha II-spectrin breakdown products have shown to be relevant markers of neurologic injury post-cardiac surgery. [24]
Clinical significance
Mutations in SPTAN1 are the cause of early infantile epileptic encephalopathy-5. [25]
Alpha II-spectrin has shown promising utility as a biomarker for brain necrosis and apoptosis in infants with congenital heart disease; breakdown products of alpha II-spectrin have been detected in the serum of neonates in the perioperative period and following open-heart surgery. [26] Elevated protein expression of alpha II-spectrin has been detected in cerebrospinal fluid in patients with Guillain–Barré syndrome. [27]
Interactions
SPTAN1 has been shown to interact with:
- Abl gene, [28]
- FANCA, [29] [30] [31]
- Fanconi anemia, complementation group C, [29] [30]
- GRIA2, [32]
- Plectin, [33] [34]
- SHANK1, [35] and
- Vimentin. [33]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000197694 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000057738 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Leto TL, Fortugno-Erikson D, Barton D, Yang-Feng TL, Francke U, Harris AS, Morrow JS, Marchesi VT, Benz EJ (February 1988). "Comparison of nonerythroid alpha-spectrin genes reveals strict homology among diverse species". Mol Cell Biol. 8 (1): 1–9. doi: 10.1128/MCB.8.1.1. PMC 363070. PMID 3336352.
- ^ Leto, T. L.; Fortugno-Erikson, D.; Barton, D.; Yang-Feng, T. L.; Francke, U.; Harris, A. S.; Morrow, J. S.; Marchesi, V. T.; Benz, E. J. (1988). "Entrez Gene: SPTAN1 spectrin, alpha, non-erythrocytic 1 (alpha-fodrin)". Molecular and Cellular Biology. 8 (1): 1–9. doi: 10.1128/MCB.8.1.1. PMC 363070. PMID 3336352.
- ^ McMahon AP, Giebelhaus DH, Champion JE, Bailes JA, Lacey S, Carritt B, Henchman SK, Moon RT (1987). "cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin". Differentiation. 34 (1): 68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. PMID 3038643.
- ^ a b c Zhang Y, Resneck WG, Lee PC, Randall WR, Bloch RJ, Ursitti JA (Jun 2010). "Characterization and expression of a heart-selective alternatively spliced variant of alpha II-spectrin, cardi+, during development in the rat". Journal of Molecular and Cellular Cardiology. 48 (6): 1050–9. doi: 10.1016/j.yjmcc.2010.01.001. PMC 3537504. PMID 20114050.
- ^ Cianci CD, Zhang Z, Pradhan D, Morrow JS (Nov 1999). "Brain and muscle express a unique alternative transcript of alphaII spectrin". Biochemistry. 38 (48): 15721–30. doi: 10.1021/bi991458k. PMID 10625438.
- ^ Ursitti JA, Kotula L, DeSilva TM, Curtis PJ, Speicher DW (Mar 1996). "Mapping the human erythrocyte beta-spectrin dimer initiation site using recombinant peptides and correlation of its phasing with the alpha-actinin dimer site". The Journal of Biological Chemistry. 271 (12): 6636–44. doi: 10.1074/jbc.271.12.6636. PMID 8636080.
- ^ a b Du A, Sanger JM, Sanger JW (Jun 2008). "Cardiac myofibrillogenesis inside intact embryonic hearts". Developmental Biology. 318 (2): 236–46. doi: 10.1016/j.ydbio.2008.03.011. PMC 2496890. PMID 18455713.
- ^ Bennett PM, Maggs AM, Baines AJ, Pinder JC (Apr 2006). "The transitional junction: a new functional subcellular domain at the intercalated disc". Molecular Biology of the Cell. 17 (4): 2091–100. doi: 10.1091/mbc.E05-12-1109. PMC 1415289. PMID 16481394.
- ^ Bennett PM, Baines AJ, Lecomte MC, Maggs AM, Pinder JC (2004). "Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils". Journal of Muscle Research and Cell Motility. 25 (2): 119–26. doi: 10.1023/b:jure.0000035892.77399.51. PMID 15360127. S2CID 10297147.
- ^ Sormunen R (Sep 1993). "Alpha-spectrin in detergent-extracted whole-mount cytoskeletons of chicken embryo heart fibroblasts". The Histochemical Journal. 25 (9): 678–86. doi: 10.1007/bf00157882. PMID 8226104. S2CID 34132236.
- ^ Bignone PA, Baines AJ (Sep 2003). "Spectrin alpha II and beta II isoforms interact with high affinity at the tetramerization site". The Biochemical Journal. 374 (Pt 3): 613–24. doi: 10.1042/BJ20030507. PMC 1223645. PMID 12820899.
- ^ a b Baines AJ, Pinder JC (1 September 2005). "The spectrin-associated cytoskeleton in mammalian heart". Frontiers in Bioscience. 10 (1–3): 3020–33. doi: 10.2741/1759. PMID 15970557.
- ^ Ursitti JA, Petrich BG, Lee PC, Resneck WG, Ye X, Yang J, Randall WR, Bloch RJ, Wang Y (Mar 2007). "Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase". Journal of Molecular and Cellular Cardiology. 42 (3): 572–81. doi: 10.1016/j.yjmcc.2006.11.018. PMC 1983066. PMID 17276456.
- ^ Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC (Jan 2009). "AlphaII-spectrin is critical for cell adhesion and cell cycle" (PDF). The Journal of Biological Chemistry. 284 (4): 2409–18. doi: 10.1074/jbc.M801324200. PMID 18978357. S2CID 18821519.
- ^ Sridharan DM, McMahon LW, Lambert MW (Nov 2006). "alphaII-Spectrin interacts with five groups of functionally important proteins in the nucleus". Cell Biology International. 30 (11): 866–78. doi: 10.1016/j.cellbi.2006.06.005. PMID 16889989. S2CID 28863657.
- ^ Nedrelow JH, Cianci CD, Morrow JS (Feb 2003). "c-Src binds alpha II spectrin's Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176". The Journal of Biological Chemistry. 278 (9): 7735–41. doi: 10.1074/jbc.M210988200. PMID 12446661.
- ^ Kennedy SP, Warren SL, Forget BG, Morrow JS (Oct 1991). "Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin". The Journal of Cell Biology. 115 (1): 267–77. doi: 10.1083/jcb.115.1.267. PMC 2289929. PMID 1833409.
- ^ Moorthy S, Chen L, Bennett V (May 2000). "Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function". The Journal of Cell Biology. 149 (4): 915–30. doi: 10.1083/jcb.149.4.915. PMC 2174577. PMID 10811831.
- ^ Wang XF, Gao GD, Liu J, Guo R, Lin YX, Chu YL, Han FC, Zhang WH, Bai YJ (2006). "Identification of differentially expressed genes induced by angiotensin II in rat cardiac fibroblasts". Clinical and Experimental Pharmacology & Physiology. 33 (1–2): 41–6. doi: 10.1111/j.1440-1681.2006.04321.x. PMID 16445697. S2CID 21008341.
- ^ Weiss ES, Wang KK, Allen JG, Blue ME, Nwakanma LU, Liu MC, Lange MS, Berrong J, Wilson MA, Gott VL, Troncoso JC, Hayes RL, Johnston MV, Baumgartner WA (Aug 2009). "Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest". The Annals of Thoracic Surgery. 88 (2): 543–50. doi: 10.1016/j.athoracsur.2009.04.016. PMC 3412404. PMID 19632410.
- ^ Writzl K, Primec ZR, Stražišar BG, Osredkar D, Pečarič-Meglič N, Kranjc BS, Nishiyama K, Matsumoto N, Saitsu H (Jun 2012). "Early onset West syndrome with severe hypomyelination and coloboma-like optic discs in a girl with SPTAN1 mutation". Epilepsia. 53 (6): e106–10. doi: 10.1111/j.1528-1167.2012.03437.x. PMID 22429196. S2CID 20216273.
- ^ Jain P, Spaeder MC, Donofrio MT, Sinha P, Jonas RA, Levy RJ (Mar 2014). "Detection of alpha II-spectrin breakdown products in the serum of neonates with congenital heart disease*". Pediatric Critical Care Medicine. 15 (3): 229–35. doi: 10.1097/PCC.0000000000000059. PMC 4059536. PMID 24395002.
- ^ Lehmensiek V, Süssmuth SD, Brettschneider J, Tauscher G, Felk S, Gillardon F, Tumani H (Apr 2007). "Proteome analysis of cerebrospinal fluid in Guillain–Barré syndrome (GBS)". Journal of Neuroimmunology. 185 (1–2): 190–4. doi: 10.1016/j.jneuroim.2007.01.022. PMID 17367871. S2CID 28987593.
- ^ Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L (May 1998). "Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton". J. Biol. Chem. 273 (22): 13681–92. doi: 10.1074/jbc.273.22.13681. PMID 9593709.
- ^ a b McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW (June 2001). "Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links". Biochemistry. 40 (24): 7025–34. doi: 10.1021/bi002917g. PMID 11401546.
- ^ a b McMahon LW, Walsh CE, Lambert MW (November 1999). "Human alpha spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex". J. Biol. Chem. 274 (46): 32904–8. doi: 10.1074/jbc.274.46.32904. PMID 10551855.
- ^ Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW (March 2003). "Nonerythroid alphaII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links". J. Cell Sci. 116 (Pt 5): 823–35. doi: 10.1242/jcs.00294. PMID 12571280.
- ^ Hirai H, Matsuda S (September 1999). "Interaction of the C-terminal domain of delta glutamate receptor with spectrin in the dendritic spines of cultured Purkinje cells". Neurosci. Res. 34 (4): 281–7. doi: 10.1016/s0168-0102(99)00061-9. PMID 10576550. S2CID 45794233.
- ^ a b Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S (July 2001). "Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin". J. Immunol. 167 (2): 641–5. doi: 10.4049/jimmunol.167.2.641. PMID 11441066.
- ^ Herrmann H, Wiche G (January 1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". J. Biol. Chem. 262 (3): 1320–5. doi: 10.1016/S0021-9258(19)75789-5. PMID 3027087.
- ^ Böckers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ (October 2001). "Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin". J. Biol. Chem. 276 (43): 40104–12. doi: 10.1074/jbc.M102454200. PMID 11509555.
Further reading
- Chow CW (1999). "Regulation and intracellular localization of the epithelial isoforms of the Na+/H+ exchangers NHE2 and NHE3". Clinical and Investigative Medicine. 22 (5): 195–206. PMID 10579058.
- Hayashi Y, Arakaki R, Ishimaru N (2003). "The role of caspase cascade on the development of primary Sjögren's syndrome". J. Med. Invest. 50 (1–2): 32–8. PMID 12630566.
- Bennett V (1979). "Immunoreactive forms of human erythrocyte ankyrin are present in diverse cells and tissues". Nature. 281 (5732): 597–9. Bibcode: 1979Natur.281..597B. doi: 10.1038/281597a0. PMID 492324. S2CID 263106.
- Frappier T, Stetzkowski-Marden F, Pradel LA (1991). "Interaction domains of neurofilament light chain and brain spectrin". Biochem. J. 275. ( Pt 2) (2): 521–7. doi: 10.1042/bj2750521. PMC 1150082. PMID 1902666.
- Bennett AF, Hayes NV, Baines AJ (1991). "Site specificity in the interactions of synapsin 1 with tubulin". Biochem. J. 276. ( Pt 3) (3): 793–9. doi: 10.1042/bj2760793. PMC 1151074. PMID 1905928.
- Davis LH, Bennett V (1990). "Mapping the binding sites of human erythrocyte ankyrin for the anion exchanger and spectrin". J. Biol. Chem. 265 (18): 10589–96. doi: 10.1016/S0021-9258(18)86987-3. PMID 2141335.
- Moon RT, McMahon AP (1990). "Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin". J. Biol. Chem. 265 (8): 4427–33. doi: 10.1016/S0021-9258(19)39582-1. PMID 2307671.
- Langley RC, Cohen CM (1986). "Association of spectrin with desmin intermediate filaments". J. Cell. Biochem. 30 (2): 101–9. doi: 10.1002/jcb.240300202. PMID 2939097. S2CID 25080821.
- Cianci CD, Giorgi M, Morrow JS (1988). "Phosphorylation of ankyrin down-regulates its cooperative interaction with spectrin and protein 3". J. Cell. Biochem. 37 (3): 301–15. doi: 10.1002/jcb.240370305. PMID 2970468. S2CID 42349239.
- Steiner JP, Bennett V (1988). "Ankyrin-independent membrane protein-binding sites for brain and erythrocyte spectrin". J. Biol. Chem. 263 (28): 14417–25. doi: 10.1016/S0021-9258(18)68236-5. PMID 2971657.
- Herrmann H, Wiche G (1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". J. Biol. Chem. 262 (3): 1320–5. doi: 10.1016/S0021-9258(19)75789-5. PMID 3027087.
- McMahon AP, Giebelhaus DH, Champion JE, Bailes JA, Lacey S, Carritt B, Henchman SK, Moon RT (1987). "cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin". Differentiation. 34 (1): 68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. PMID 3038643.
- Frappier T, Regnouf F, Pradel LA (1988). "Binding of brain spectrin to the 70-kDa neurofilament subunit protein". Eur. J. Biochem. 169 (3): 651–7. doi: 10.1111/j.1432-1033.1987.tb13657.x. PMID 3121319.
- McMahon AP, Moon RT (1988). "Structure and evolution of a non-erythroid spectrin, human alpha-fodrin". Biochem. Soc. Trans. 15 (5): 804–7. doi: 10.1042/bst0150804. PMID 3691949.
- Lundberg S, Björk J, Löfvenberg L, Backman L (1995). "Cloning, expression and characterization of two putative calcium-binding sites in human non-erythroid alpha-spectrin". Eur. J. Biochem. 230 (2): 658–65. doi: 10.1111/j.1432-1033.1995.0658h.x. PMID 7607240.
- Hughes CA, Bennett V (1995). "Adducin: a physical model with implications for function in assembly of spectrin-actin complexes". J. Biol. Chem. 270 (32): 18990–6. doi: 10.1074/jbc.270.32.18990. PMID 7642559.
- Gregorio CC, Repasky EA, Fowler VM, Black JD (1994). "Dynamic properties of ankyrin in T lymphocytes: colocalization with spectrin and protein kinase C beta". J. Cell Biol. 125 (2): 345–58. doi: 10.1083/jcb.125.2.345. PMC 2120020. PMID 8163551.
- Li X, Bennett V (1996). "Identification of the spectrin subunit and domains required for formation of spectrin/adducin/actin complexes". J. Biol. Chem. 271 (26): 15695–702. doi: 10.1074/jbc.271.26.15695. PMID 8663089.
- Stabach PR, Cianci CD, Glantz SB, Zhang Z, Morrow JS (1997). "Site-directed mutagenesis of alpha II spectrin at codon 1175 modulates its mu-calpain susceptibility". Biochemistry. 36 (1): 57–65. doi: 10.1021/bi962034i. PMID 8993318.
| SPTAN1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SPTAN1, EIEE5, NEAS, SPTA2, spectrin alpha, non-erythrocytic 1, DEE5 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 182810; MGI: 98386; HomoloGene: 2353; GeneCards: SPTAN1; OMA: SPTAN1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
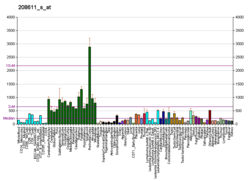
Alpha II-spectrin, also known as Spectrin alpha chain, brain is a protein that in humans is encoded by the SPTAN1 gene. [5] [6] [7] Alpha II-spectrin is expressed in a variety of tissues, and is highly expressed in cardiac muscle at Z-disc structures, costameres and at the sarcolemma membrane. Mutations in alpha II-spectrin have been associated with early infantile epileptic encephalopathy-5, and alpha II-spectrin may be a valuable biomarker for Guillain–Barré syndrome and infantile congenital heart disease.
Structure
Alternate splicing of alpha II-spectrin has been documented and results in multiple transcript variants; specifically, cardiomyocytes have four identified alpha II-spectrin splice variants. [8] [9] As opposed to alpha I-spectrin that is principally found in erythrocytes, [10] alpha II-spectrin is expressed in most tissues. In cardiac tissue, alpha II-spectrin is found in myocytes at Z-discs, costameres, and the sarcolemma membrane, [11] [12] [13] and in cardiac fibroblasts along the surface of the cytoskeletal network. [14] Alpha II-spectrin most commonly exists in a heterodimer with alpha II and beta II spectrin subunits; and dimers typically self-associate and heterotetramerize. [5] [15] [16]
Function
The spectrins are a family of widely distributed cytoskeletal proteins which are involved in actin crosslinking, cell adhesion, intercellular communication and cell cycle regulation. [17] [18] [19] Though a role in cardiac muscle is not well understood, it is likely that alpha II-spectrin is involved in organizing sub- sarcolemmal domains and stabilizing sarcolemmal membranes against the stresses associated with continuous cardiac contraction. [16] Functional diversity of alpha II-spectrin is manifest through its four splice variants. First, a cardiac-specific, 21 amino acid sequence insert in the 21st spectrin repeat, termed alpha II-cardi+, was identified as an insert that modulates affinity of alpha II-spectrin for binding beta-spectrins and regulates myocyte growth and differentiation. [8] Secondly, another insert of 20 amino acids in the 10th spectrin repeat, termed SH3i+, contains protein kinase A and protein kinase C phosphorylation sites and modulates Ca2+-dependent cleavage of spectrin and protein-protein interaction properties. [20] Thirdly, an insert of five amino acids in the fifteenth spectrin motif bears a highly antigenic epitope resembling an ankyrin-like p53 binding protein binding site. [8] [21] Fourthly, a six amino acid insert in the twenty-first spectrin motif with unknown function has been reported. [11] [22]
Alpha II-spectrin gene expression has been shown to be upregulated in cardiac fibroblasts in response to Angiotensin II-induced cardiac remodeling. [23]
In animal models of disease and injury, alpha II-spectrin has been implicated in diverse functions. In a canine model of hypothermic circulatory arrest, alpha II-spectrin breakdown products have shown to be relevant markers of neurologic injury post-cardiac surgery. [24]
Clinical significance
Mutations in SPTAN1 are the cause of early infantile epileptic encephalopathy-5. [25]
Alpha II-spectrin has shown promising utility as a biomarker for brain necrosis and apoptosis in infants with congenital heart disease; breakdown products of alpha II-spectrin have been detected in the serum of neonates in the perioperative period and following open-heart surgery. [26] Elevated protein expression of alpha II-spectrin has been detected in cerebrospinal fluid in patients with Guillain–Barré syndrome. [27]
Interactions
SPTAN1 has been shown to interact with:
- Abl gene, [28]
- FANCA, [29] [30] [31]
- Fanconi anemia, complementation group C, [29] [30]
- GRIA2, [32]
- Plectin, [33] [34]
- SHANK1, [35] and
- Vimentin. [33]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000197694 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000057738 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Leto TL, Fortugno-Erikson D, Barton D, Yang-Feng TL, Francke U, Harris AS, Morrow JS, Marchesi VT, Benz EJ (February 1988). "Comparison of nonerythroid alpha-spectrin genes reveals strict homology among diverse species". Mol Cell Biol. 8 (1): 1–9. doi: 10.1128/MCB.8.1.1. PMC 363070. PMID 3336352.
- ^ Leto, T. L.; Fortugno-Erikson, D.; Barton, D.; Yang-Feng, T. L.; Francke, U.; Harris, A. S.; Morrow, J. S.; Marchesi, V. T.; Benz, E. J. (1988). "Entrez Gene: SPTAN1 spectrin, alpha, non-erythrocytic 1 (alpha-fodrin)". Molecular and Cellular Biology. 8 (1): 1–9. doi: 10.1128/MCB.8.1.1. PMC 363070. PMID 3336352.
- ^ McMahon AP, Giebelhaus DH, Champion JE, Bailes JA, Lacey S, Carritt B, Henchman SK, Moon RT (1987). "cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin". Differentiation. 34 (1): 68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. PMID 3038643.
- ^ a b c Zhang Y, Resneck WG, Lee PC, Randall WR, Bloch RJ, Ursitti JA (Jun 2010). "Characterization and expression of a heart-selective alternatively spliced variant of alpha II-spectrin, cardi+, during development in the rat". Journal of Molecular and Cellular Cardiology. 48 (6): 1050–9. doi: 10.1016/j.yjmcc.2010.01.001. PMC 3537504. PMID 20114050.
- ^ Cianci CD, Zhang Z, Pradhan D, Morrow JS (Nov 1999). "Brain and muscle express a unique alternative transcript of alphaII spectrin". Biochemistry. 38 (48): 15721–30. doi: 10.1021/bi991458k. PMID 10625438.
- ^ Ursitti JA, Kotula L, DeSilva TM, Curtis PJ, Speicher DW (Mar 1996). "Mapping the human erythrocyte beta-spectrin dimer initiation site using recombinant peptides and correlation of its phasing with the alpha-actinin dimer site". The Journal of Biological Chemistry. 271 (12): 6636–44. doi: 10.1074/jbc.271.12.6636. PMID 8636080.
- ^ a b Du A, Sanger JM, Sanger JW (Jun 2008). "Cardiac myofibrillogenesis inside intact embryonic hearts". Developmental Biology. 318 (2): 236–46. doi: 10.1016/j.ydbio.2008.03.011. PMC 2496890. PMID 18455713.
- ^ Bennett PM, Maggs AM, Baines AJ, Pinder JC (Apr 2006). "The transitional junction: a new functional subcellular domain at the intercalated disc". Molecular Biology of the Cell. 17 (4): 2091–100. doi: 10.1091/mbc.E05-12-1109. PMC 1415289. PMID 16481394.
- ^ Bennett PM, Baines AJ, Lecomte MC, Maggs AM, Pinder JC (2004). "Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils". Journal of Muscle Research and Cell Motility. 25 (2): 119–26. doi: 10.1023/b:jure.0000035892.77399.51. PMID 15360127. S2CID 10297147.
- ^ Sormunen R (Sep 1993). "Alpha-spectrin in detergent-extracted whole-mount cytoskeletons of chicken embryo heart fibroblasts". The Histochemical Journal. 25 (9): 678–86. doi: 10.1007/bf00157882. PMID 8226104. S2CID 34132236.
- ^ Bignone PA, Baines AJ (Sep 2003). "Spectrin alpha II and beta II isoforms interact with high affinity at the tetramerization site". The Biochemical Journal. 374 (Pt 3): 613–24. doi: 10.1042/BJ20030507. PMC 1223645. PMID 12820899.
- ^ a b Baines AJ, Pinder JC (1 September 2005). "The spectrin-associated cytoskeleton in mammalian heart". Frontiers in Bioscience. 10 (1–3): 3020–33. doi: 10.2741/1759. PMID 15970557.
- ^ Ursitti JA, Petrich BG, Lee PC, Resneck WG, Ye X, Yang J, Randall WR, Bloch RJ, Wang Y (Mar 2007). "Role of an alternatively spliced form of alphaII-spectrin in localization of connexin 43 in cardiomyocytes and regulation by stress-activated protein kinase". Journal of Molecular and Cellular Cardiology. 42 (3): 572–81. doi: 10.1016/j.yjmcc.2006.11.018. PMC 1983066. PMID 17276456.
- ^ Metral S, Machnicka B, Bigot S, Colin Y, Dhermy D, Lecomte MC (Jan 2009). "AlphaII-spectrin is critical for cell adhesion and cell cycle" (PDF). The Journal of Biological Chemistry. 284 (4): 2409–18. doi: 10.1074/jbc.M801324200. PMID 18978357. S2CID 18821519.
- ^ Sridharan DM, McMahon LW, Lambert MW (Nov 2006). "alphaII-Spectrin interacts with five groups of functionally important proteins in the nucleus". Cell Biology International. 30 (11): 866–78. doi: 10.1016/j.cellbi.2006.06.005. PMID 16889989. S2CID 28863657.
- ^ Nedrelow JH, Cianci CD, Morrow JS (Feb 2003). "c-Src binds alpha II spectrin's Src homology 3 (SH3) domain and blocks calpain susceptibility by phosphorylating Tyr1176". The Journal of Biological Chemistry. 278 (9): 7735–41. doi: 10.1074/jbc.M210988200. PMID 12446661.
- ^ Kennedy SP, Warren SL, Forget BG, Morrow JS (Oct 1991). "Ankyrin binds to the 15th repetitive unit of erythroid and nonerythroid beta-spectrin". The Journal of Cell Biology. 115 (1): 267–77. doi: 10.1083/jcb.115.1.267. PMC 2289929. PMID 1833409.
- ^ Moorthy S, Chen L, Bennett V (May 2000). "Caenorhabditis elegans beta-G spectrin is dispensable for establishment of epithelial polarity, but essential for muscular and neuronal function". The Journal of Cell Biology. 149 (4): 915–30. doi: 10.1083/jcb.149.4.915. PMC 2174577. PMID 10811831.
- ^ Wang XF, Gao GD, Liu J, Guo R, Lin YX, Chu YL, Han FC, Zhang WH, Bai YJ (2006). "Identification of differentially expressed genes induced by angiotensin II in rat cardiac fibroblasts". Clinical and Experimental Pharmacology & Physiology. 33 (1–2): 41–6. doi: 10.1111/j.1440-1681.2006.04321.x. PMID 16445697. S2CID 21008341.
- ^ Weiss ES, Wang KK, Allen JG, Blue ME, Nwakanma LU, Liu MC, Lange MS, Berrong J, Wilson MA, Gott VL, Troncoso JC, Hayes RL, Johnston MV, Baumgartner WA (Aug 2009). "Alpha II-spectrin breakdown products serve as novel markers of brain injury severity in a canine model of hypothermic circulatory arrest". The Annals of Thoracic Surgery. 88 (2): 543–50. doi: 10.1016/j.athoracsur.2009.04.016. PMC 3412404. PMID 19632410.
- ^ Writzl K, Primec ZR, Stražišar BG, Osredkar D, Pečarič-Meglič N, Kranjc BS, Nishiyama K, Matsumoto N, Saitsu H (Jun 2012). "Early onset West syndrome with severe hypomyelination and coloboma-like optic discs in a girl with SPTAN1 mutation". Epilepsia. 53 (6): e106–10. doi: 10.1111/j.1528-1167.2012.03437.x. PMID 22429196. S2CID 20216273.
- ^ Jain P, Spaeder MC, Donofrio MT, Sinha P, Jonas RA, Levy RJ (Mar 2014). "Detection of alpha II-spectrin breakdown products in the serum of neonates with congenital heart disease*". Pediatric Critical Care Medicine. 15 (3): 229–35. doi: 10.1097/PCC.0000000000000059. PMC 4059536. PMID 24395002.
- ^ Lehmensiek V, Süssmuth SD, Brettschneider J, Tauscher G, Felk S, Gillardon F, Tumani H (Apr 2007). "Proteome analysis of cerebrospinal fluid in Guillain–Barré syndrome (GBS)". Journal of Neuroimmunology. 185 (1–2): 190–4. doi: 10.1016/j.jneuroim.2007.01.022. PMID 17367871. S2CID 28987593.
- ^ Ziemnicka-Kotula D, Xu J, Gu H, Potempska A, Kim KS, Jenkins EC, Trenkner E, Kotula L (May 1998). "Identification of a candidate human spectrin Src homology 3 domain-binding protein suggests a general mechanism of association of tyrosine kinases with the spectrin-based membrane skeleton". J. Biol. Chem. 273 (22): 13681–92. doi: 10.1074/jbc.273.22.13681. PMID 9593709.
- ^ a b McMahon LW, Sangerman J, Goodman SR, Kumaresan K, Lambert MW (June 2001). "Human alpha spectrin II and the FANCA, FANCC, and FANCG proteins bind to DNA containing psoralen interstrand cross-links". Biochemistry. 40 (24): 7025–34. doi: 10.1021/bi002917g. PMID 11401546.
- ^ a b McMahon LW, Walsh CE, Lambert MW (November 1999). "Human alpha spectrin II and the Fanconi anemia proteins FANCA and FANCC interact to form a nuclear complex". J. Biol. Chem. 274 (46): 32904–8. doi: 10.1074/jbc.274.46.32904. PMID 10551855.
- ^ Sridharan D, Brown M, Lambert WC, McMahon LW, Lambert MW (March 2003). "Nonerythroid alphaII spectrin is required for recruitment of FANCA and XPF to nuclear foci induced by DNA interstrand cross-links". J. Cell Sci. 116 (Pt 5): 823–35. doi: 10.1242/jcs.00294. PMID 12571280.
- ^ Hirai H, Matsuda S (September 1999). "Interaction of the C-terminal domain of delta glutamate receptor with spectrin in the dendritic spines of cultured Purkinje cells". Neurosci. Res. 34 (4): 281–7. doi: 10.1016/s0168-0102(99)00061-9. PMID 10576550. S2CID 45794233.
- ^ a b Brown MJ, Hallam JA, Liu Y, Yamada KM, Shaw S (July 2001). "Cutting edge: integration of human T lymphocyte cytoskeleton by the cytolinker plectin". J. Immunol. 167 (2): 641–5. doi: 10.4049/jimmunol.167.2.641. PMID 11441066.
- ^ Herrmann H, Wiche G (January 1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". J. Biol. Chem. 262 (3): 1320–5. doi: 10.1016/S0021-9258(19)75789-5. PMID 3027087.
- ^ Böckers TM, Mameza MG, Kreutz MR, Bockmann J, Weise C, Buck F, Richter D, Gundelfinger ED, Kreienkamp HJ (October 2001). "Synaptic scaffolding proteins in rat brain. Ankyrin repeats of the multidomain Shank protein family interact with the cytoskeletal protein alpha-fodrin". J. Biol. Chem. 276 (43): 40104–12. doi: 10.1074/jbc.M102454200. PMID 11509555.
Further reading
- Chow CW (1999). "Regulation and intracellular localization of the epithelial isoforms of the Na+/H+ exchangers NHE2 and NHE3". Clinical and Investigative Medicine. 22 (5): 195–206. PMID 10579058.
- Hayashi Y, Arakaki R, Ishimaru N (2003). "The role of caspase cascade on the development of primary Sjögren's syndrome". J. Med. Invest. 50 (1–2): 32–8. PMID 12630566.
- Bennett V (1979). "Immunoreactive forms of human erythrocyte ankyrin are present in diverse cells and tissues". Nature. 281 (5732): 597–9. Bibcode: 1979Natur.281..597B. doi: 10.1038/281597a0. PMID 492324. S2CID 263106.
- Frappier T, Stetzkowski-Marden F, Pradel LA (1991). "Interaction domains of neurofilament light chain and brain spectrin". Biochem. J. 275. ( Pt 2) (2): 521–7. doi: 10.1042/bj2750521. PMC 1150082. PMID 1902666.
- Bennett AF, Hayes NV, Baines AJ (1991). "Site specificity in the interactions of synapsin 1 with tubulin". Biochem. J. 276. ( Pt 3) (3): 793–9. doi: 10.1042/bj2760793. PMC 1151074. PMID 1905928.
- Davis LH, Bennett V (1990). "Mapping the binding sites of human erythrocyte ankyrin for the anion exchanger and spectrin". J. Biol. Chem. 265 (18): 10589–96. doi: 10.1016/S0021-9258(18)86987-3. PMID 2141335.
- Moon RT, McMahon AP (1990). "Generation of diversity in nonerythroid spectrins. Multiple polypeptides are predicted by sequence analysis of cDNAs encompassing the coding region of human nonerythroid alpha-spectrin". J. Biol. Chem. 265 (8): 4427–33. doi: 10.1016/S0021-9258(19)39582-1. PMID 2307671.
- Langley RC, Cohen CM (1986). "Association of spectrin with desmin intermediate filaments". J. Cell. Biochem. 30 (2): 101–9. doi: 10.1002/jcb.240300202. PMID 2939097. S2CID 25080821.
- Cianci CD, Giorgi M, Morrow JS (1988). "Phosphorylation of ankyrin down-regulates its cooperative interaction with spectrin and protein 3". J. Cell. Biochem. 37 (3): 301–15. doi: 10.1002/jcb.240370305. PMID 2970468. S2CID 42349239.
- Steiner JP, Bennett V (1988). "Ankyrin-independent membrane protein-binding sites for brain and erythrocyte spectrin". J. Biol. Chem. 263 (28): 14417–25. doi: 10.1016/S0021-9258(18)68236-5. PMID 2971657.
- Herrmann H, Wiche G (1987). "Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin". J. Biol. Chem. 262 (3): 1320–5. doi: 10.1016/S0021-9258(19)75789-5. PMID 3027087.
- McMahon AP, Giebelhaus DH, Champion JE, Bailes JA, Lacey S, Carritt B, Henchman SK, Moon RT (1987). "cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin". Differentiation. 34 (1): 68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. PMID 3038643.
- Frappier T, Regnouf F, Pradel LA (1988). "Binding of brain spectrin to the 70-kDa neurofilament subunit protein". Eur. J. Biochem. 169 (3): 651–7. doi: 10.1111/j.1432-1033.1987.tb13657.x. PMID 3121319.
- McMahon AP, Moon RT (1988). "Structure and evolution of a non-erythroid spectrin, human alpha-fodrin". Biochem. Soc. Trans. 15 (5): 804–7. doi: 10.1042/bst0150804. PMID 3691949.
- Lundberg S, Björk J, Löfvenberg L, Backman L (1995). "Cloning, expression and characterization of two putative calcium-binding sites in human non-erythroid alpha-spectrin". Eur. J. Biochem. 230 (2): 658–65. doi: 10.1111/j.1432-1033.1995.0658h.x. PMID 7607240.
- Hughes CA, Bennett V (1995). "Adducin: a physical model with implications for function in assembly of spectrin-actin complexes". J. Biol. Chem. 270 (32): 18990–6. doi: 10.1074/jbc.270.32.18990. PMID 7642559.
- Gregorio CC, Repasky EA, Fowler VM, Black JD (1994). "Dynamic properties of ankyrin in T lymphocytes: colocalization with spectrin and protein kinase C beta". J. Cell Biol. 125 (2): 345–58. doi: 10.1083/jcb.125.2.345. PMC 2120020. PMID 8163551.
- Li X, Bennett V (1996). "Identification of the spectrin subunit and domains required for formation of spectrin/adducin/actin complexes". J. Biol. Chem. 271 (26): 15695–702. doi: 10.1074/jbc.271.26.15695. PMID 8663089.
- Stabach PR, Cianci CD, Glantz SB, Zhang Z, Morrow JS (1997). "Site-directed mutagenesis of alpha II spectrin at codon 1175 modulates its mu-calpain susceptibility". Biochemistry. 36 (1): 57–65. doi: 10.1021/bi962034i. PMID 8993318.