| |
| Names | |
|---|---|
|
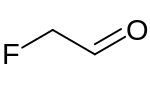
Preferred IUPAC name
Fluoroacetaldehyde | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C2H3FO | |
| Molar mass | 62.043 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluoroacetaldehyde is a metabolic precursor of both fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. [1]
- ^ Steven J. Moss; Cormac D. Murphy; John T. G. Hamilton; W. Colin McRoberts; David O’Hagan; Christoph Schaffrath; David B. Harper (2000). "Fluoroacetaldehyde: a precursor of both fluoroacetateand 4-fluorothreonine in Streptomyces cattleya". Chemical Communications (22): 2281–2282. doi: 10.1039/B007261N.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
Fluoroacetaldehyde | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C2H3FO | |
| Molar mass | 62.043 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluoroacetaldehyde is a metabolic precursor of both fluoroacetate and 4-fluorothreonine in Streptomyces cattleya. [1]
- ^ Steven J. Moss; Cormac D. Murphy; John T. G. Hamilton; W. Colin McRoberts; David O’Hagan; Christoph Schaffrath; David B. Harper (2000). "Fluoroacetaldehyde: a precursor of both fluoroacetateand 4-fluorothreonine in Streptomyces cattleya". Chemical Communications (22): 2281–2282. doi: 10.1039/B007261N.