| fascin homolog 1 | |||||||
|---|---|---|---|---|---|---|---|
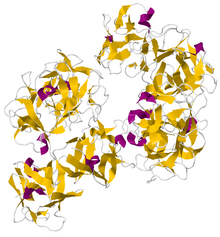
Crystallographic structure of dimeric human fascin 1.
[1] | |||||||
| Identifiers | |||||||
| Symbol | FSCN1 | ||||||
| Alt. symbols | SNL | ||||||
| NCBI gene | 6624 | ||||||
| HGNC | 11148 | ||||||
| OMIM | 602689 | ||||||
| RefSeq | NM_003088 | ||||||
| UniProt | Q16658 | ||||||
| Other data | |||||||
| Locus | Chr. 7 p22 | ||||||
| |||||||
| fascin homolog, retinal | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FSCN2 | ||||||
| NCBI gene | 25794 | ||||||
| HGNC | 3960 | ||||||
| OMIM | 607643 | ||||||
| RefSeq | NM_012418 | ||||||
| UniProt | O14926 | ||||||
| Other data | |||||||
| Locus | Chr. 17 q25 | ||||||
| |||||||
| fascin homolog 3, testicular | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FSCN3 | ||||||
| NCBI gene | 29999 | ||||||
| HGNC | 3961 | ||||||
| RefSeq | NM_020369 | ||||||
| UniProt | Q9NQT6 | ||||||
| Other data | |||||||
| Locus | Chr. 7 q31.3 | ||||||
| |||||||
Fascin is an actin bundling protein.
Species and tissue distribution
It is a 54–58 kilodalton monomeric actin filament bundling protein originally isolated from sea urchin egg but also found in Drosophila [2] and vertebrates, [3] including humans. [4] Fascin (from the Latin for bundle) is spaced at 11 nanometre intervals along the filament. The bundles in cross section are seen to be hexagonally packed, and the longitudinal spacing is compatible with a model where fascin cross-links at alternating 4 and 5 actins. [5] It is calcium insensitive and monomeric. Three forms of fascin are found in vertebrates: Fascin1, widely found in the nervous system and elsewhere; fascin2 found in the retinal photoreceptor cells; fascin3, which is only found in the testes. [6] [7]
Function
Fascin binds beta-catenin, [8] and colocalizes with it at the leading edges and borders of epithelial and endothelial cells. The role of Fascin in regulating cytoskeletal structures for the maintenance of cell adhesion, coordinating motility and invasion through interactions with signalling pathways is an active area of research especially from the cancer biology perspective. [6] [7] Fascin localizes to actin-rich protrusions at the cell surface called filopodia. Recent study shows that fascin also localizes to invadopodia, membrane protrusions formed at the adherent cell surface that facilitate extracellular matrix (ECM) invasion, this provide a potential molecular mechanism for how fascin increases the invasiveness of cancer cells since fascin expression is upregulated in a spectrum of cancers. [9] Studies have also shown that Fascin plays a major role in immune suppression. T regulatory cell adhesion to antigen presenting dendritic cell causes sequestration of Fascin-1, an actin-bundling protein essential for immunological synapse formation, and skews Fascin-1–dependent actin polarization in antigen presenting dendritic cells toward the T reg cell adhesion zone. Although it is reversible upon T regulatory cell disengagement, this sequestration of essential cytoskeletal components causes a lethargic state of dendritic cells, leading to reduced T cell priming. This suggests Treg-mediated suppression of antigen presenting cells is a multi-step process. In addition to CTLA-4 CD80/CD86 interaction fascin dependent polarization of cytoskeleton towards dendritic cell Treg immune synapse play a pivotal role. [10] In normal tissue, inflammation and the immune response would be limited by secretion of TGF-β. TGF-β on the one hand induces fascin expression, but on the other hand, restricts activity of transcription factor NF-κB. This results to limited fascin expression and allows tissue to rebuild epithelial barriers. In cancer, instead, TGF-β does not restrict NF-κB activity, and both can increase fascin expression, disrupting tissue structure and function. [11]
Clinical significance
Abnormal fascin expression or function has been implicated in breast cancer, [12] colon cancer, [13] [14] esophageal squamous cell carcinoma, [15] gallbladder cancer, [16] pancreatic cancer, [17] and prostate cancer. [18] It is also helpful in identifying Hodgkin cells.
Structure
Fascin is a structural protein found in mesenchyme, nervous, and retinal tissue and is used in the bundling of actin molecules. [19]
The structure of human fascin has been determined to a resolution of 1.8 Å (PDBID 3LLP) and reveals an arrangement of four tandem beta-trefoil domains that form a two lobed structure with pseudo 2-fold symmetry. It is stabilized by a hydrophobic core and a hydrophilic surface since it is often found inside cell cytoplasm in the formation of filopodia. [1]
References
- ^ a b PDB: 1DFC; Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, Bathe M (July 2010). "Structure, evolutionary conservation, and conformational dynamics of Homo sapiens fascin-1, an F-actin crosslinking protein". J. Mol. Biol. 400 (3): 589–604. doi: 10.1016/j.jmb.2010.04.043. PMC 7141155. PMID 20434460.
- ^ Bryan J, Edwards R, Matsudaira P, Otto J, Wulfkuhle J (October 1993). "Fascin, an echinoid actin-bundling protein, is a homolog of the Drosophila singed gene product". Proceedings of the National Academy of Sciences of the United States of America. 90 (19): 9115–9119. Bibcode: 1993PNAS...90.9115B. doi: 10.1073/pnas.90.19.9115. ISSN 0027-8424. PMC 47512. PMID 8415664.
- ^ Edwards RA, Bryan J (1995). "Fascins, a family of actin bundling proteins". Cell Motility and the Cytoskeleton. 32 (1). John Wiley & Sons: 1–9. doi: 10.1002/cm.970320102. ISSN 0886-1544. PMID 8674129.
- ^ Yamashiro-Matsumura S, Matsumura F (April 1985). "Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells". Journal of Biological Chemistry. 260 (8): 5087–5097. doi: 10.1016/S0021-9258(18)89183-9. ISSN 0021-9258. PMID 3886649.
- ^ Bryan J, Kane RE (October 1978). "Separation and interaction of the major components of sea urchin actin gel". Journal of Molecular Biology. 125 (2): 207–224. doi: 10.1016/0022-2836(78)90345-5. ISSN 0022-2836. PMID 731692.
- ^ a b Adams JC (October 2004). "Roles of fascin in cell adhesion and motility". Current Opinion in Cell Biology. 16 (5): 590–596. doi: 10.1016/j.ceb.2004.07.009. ISSN 0955-0674. PMID 15363811.
- ^ a b Hashimoto Y, Skacel M, Adams JC (September 2005). "Roles of fascin in human carcinoma motility and signaling: Prospects for a novel biomarker?". The International Journal of Biochemistry & Cell Biology. 37 (9). Elsevier: 1787–1804. doi: 10.1016/j.biocel.2005.05.004. ISSN 1357-2725. PMID 16002322.
- ^ Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD (September 1996). "beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex". Journal of Cell Biology. 134 (5). Rockefeller University Press: 1271–1281. doi: 10.1083/jcb.134.5.1271. ISSN 0021-9525. PMC 2120989. PMID 8794867.
- ^ Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM (Feb 2010). "The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion". Curr. Biol. 20 (4): 339–45. Bibcode: 2010CBio...20..339L. doi: 10.1016/j.cub.2009.12.035. PMC 3163294. PMID 20137952.
- ^ Chen J, Ganguly A, Mucsi AD, Meng J, Yan J, Detampel P, Munro F, Zhang Z, Wu M, Hari A, Stenner MD, Zheng W, Kubes P, Xia T, Amrein MW, Qi H, Shi Y (2017-01-12). "Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy". Journal of Experimental Medicine. 214 (2): –20160620. doi: 10.1084/jem.20160620. eISSN 1540-9538. ISSN 0022-1007. PMC 5294852. PMID 28082358.
- ^ Vlahopoulos SA, Cen O, Hengen N, Agan J, Moschovi M, Critselis E, Adamaki M, Bacopoulou F, Copland JA, Boldogh I, Karin M, Chrousos GP (20 June 2015). "Dynamic aberrant NF-κB spurs tumorigenesis: A new model encompassing the microenvironment". Cytokine & Growth Factor Reviews. 26 (4): 389–403. doi: 10.1016/j.cytogfr.2015.06.001. PMC 4526340. PMID 26119834.
- ^ Grothey A, Hashizume R, Sahin AA, McCrea PD (2000). "Fascin, an actin-bundling protein associated with cell motility, is upregulated in hormone receptor negative breast cancer". British Journal of Cancer. 83 (7): 870–873. doi: 10.1054/bjoc.2000.1395. ISSN 0007-0920. PMC 2374674. PMID 10970687.
- ^ Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, Farthing MJ, Pignatelli M, Adams JC (January 2003). "Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro". American Journal of Pathology. 162 (1). American Society for Investigative Pathology: 69–80. doi: 10.1016/S0002-9440(10)63799-6. ISSN 0002-9440. PMC 1851132. PMID 12507891.
- ^ Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Laé M, Louvard D, Ben-Ze'ev A, Robine S (July 2007). "Fascin, a Novel Target of β-Catenin-TCF Signaling, Is Expressed at the Invasive Front of Human Colon Cancer". Cancer Research. 67 (14): 6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. ISSN 0008-5472. PMID 17638895.
- ^ Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S, Higashiyama M, Watanabe G, Imamura M, Shimada Y (April 2005). "Prognostic Significance of Fascin Overexpression in Human Esophageal Squamous Cell Carcinoma". Clinical Cancer Research. 11 (7). American Association for Cancer Research: 2597–2605. doi: 10.1158/1078-0432.CCR-04-1378. ISSN 1078-0432. PMID 15814639.
- ^ Roh YH, Kim YH, Choi HJ, Lee KE, Roh MS (March 2009). "Fascin overexpression correlates with positive thrombospondin-1 and syndecan-1 expressions and a more aggressive clinical course in patients with gallbladder cancer". Journal of Hepato-Biliary-Pancreatic Surgery. 16 (3). Springer International: 315–21. doi: 10.1007/s00534-009-0046-1. ISSN 0944-1166. PMID 19259612.
- ^ Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, Jamieson NB, MacKay CJ, Carter CR, Leung HY, Yamashiro S, Blyth K, Sansom OJ, Machesky LM (2014). "Fascin Is Regulated by Slug, Promotes Progression of Pancreatic Cancer in Mice, and Is Associated With Patient Outcomes". Gastroenterology. 146 (5): 1386–1396.e17. doi: 10.1053/j.gastro.2014.01.046. ISSN 0016-5085. PMC 4000441. PMID 24462734.
- ^ Darnel AD, Behmoaram E, Vollmer RT, Corcos J, Bijian K, Sircar K, Su J, Jiao J, Alaoui-Jamali MA, Bismar TA (February 2009). "Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer". Clinical Cancer Research. 15 (4). American Association for Cancer Research: 1376–1383. doi: 10.1158/1078-0432.CCR-08-1789. ISSN 1078-0432. PMID 19228738.
- ^ Jayo A, Parsons M (October 2010). "Fascin: a key regulator of cytoskeletal dynamics". Int. J. Biochem. Cell Biol. 42 (10): 1614–7. doi: 10.1016/j.biocel.2010.06.019. PMID 20601080.
External links
- fascin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
| fascin homolog 1 | |||||||
|---|---|---|---|---|---|---|---|

Crystallographic structure of dimeric human fascin 1.
[1] | |||||||
| Identifiers | |||||||
| Symbol | FSCN1 | ||||||
| Alt. symbols | SNL | ||||||
| NCBI gene | 6624 | ||||||
| HGNC | 11148 | ||||||
| OMIM | 602689 | ||||||
| RefSeq | NM_003088 | ||||||
| UniProt | Q16658 | ||||||
| Other data | |||||||
| Locus | Chr. 7 p22 | ||||||
| |||||||
| fascin homolog, retinal | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FSCN2 | ||||||
| NCBI gene | 25794 | ||||||
| HGNC | 3960 | ||||||
| OMIM | 607643 | ||||||
| RefSeq | NM_012418 | ||||||
| UniProt | O14926 | ||||||
| Other data | |||||||
| Locus | Chr. 17 q25 | ||||||
| |||||||
| fascin homolog 3, testicular | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Symbol | FSCN3 | ||||||
| NCBI gene | 29999 | ||||||
| HGNC | 3961 | ||||||
| RefSeq | NM_020369 | ||||||
| UniProt | Q9NQT6 | ||||||
| Other data | |||||||
| Locus | Chr. 7 q31.3 | ||||||
| |||||||
Fascin is an actin bundling protein.
Species and tissue distribution
It is a 54–58 kilodalton monomeric actin filament bundling protein originally isolated from sea urchin egg but also found in Drosophila [2] and vertebrates, [3] including humans. [4] Fascin (from the Latin for bundle) is spaced at 11 nanometre intervals along the filament. The bundles in cross section are seen to be hexagonally packed, and the longitudinal spacing is compatible with a model where fascin cross-links at alternating 4 and 5 actins. [5] It is calcium insensitive and monomeric. Three forms of fascin are found in vertebrates: Fascin1, widely found in the nervous system and elsewhere; fascin2 found in the retinal photoreceptor cells; fascin3, which is only found in the testes. [6] [7]
Function
Fascin binds beta-catenin, [8] and colocalizes with it at the leading edges and borders of epithelial and endothelial cells. The role of Fascin in regulating cytoskeletal structures for the maintenance of cell adhesion, coordinating motility and invasion through interactions with signalling pathways is an active area of research especially from the cancer biology perspective. [6] [7] Fascin localizes to actin-rich protrusions at the cell surface called filopodia. Recent study shows that fascin also localizes to invadopodia, membrane protrusions formed at the adherent cell surface that facilitate extracellular matrix (ECM) invasion, this provide a potential molecular mechanism for how fascin increases the invasiveness of cancer cells since fascin expression is upregulated in a spectrum of cancers. [9] Studies have also shown that Fascin plays a major role in immune suppression. T regulatory cell adhesion to antigen presenting dendritic cell causes sequestration of Fascin-1, an actin-bundling protein essential for immunological synapse formation, and skews Fascin-1–dependent actin polarization in antigen presenting dendritic cells toward the T reg cell adhesion zone. Although it is reversible upon T regulatory cell disengagement, this sequestration of essential cytoskeletal components causes a lethargic state of dendritic cells, leading to reduced T cell priming. This suggests Treg-mediated suppression of antigen presenting cells is a multi-step process. In addition to CTLA-4 CD80/CD86 interaction fascin dependent polarization of cytoskeleton towards dendritic cell Treg immune synapse play a pivotal role. [10] In normal tissue, inflammation and the immune response would be limited by secretion of TGF-β. TGF-β on the one hand induces fascin expression, but on the other hand, restricts activity of transcription factor NF-κB. This results to limited fascin expression and allows tissue to rebuild epithelial barriers. In cancer, instead, TGF-β does not restrict NF-κB activity, and both can increase fascin expression, disrupting tissue structure and function. [11]
Clinical significance
Abnormal fascin expression or function has been implicated in breast cancer, [12] colon cancer, [13] [14] esophageal squamous cell carcinoma, [15] gallbladder cancer, [16] pancreatic cancer, [17] and prostate cancer. [18] It is also helpful in identifying Hodgkin cells.
Structure
Fascin is a structural protein found in mesenchyme, nervous, and retinal tissue and is used in the bundling of actin molecules. [19]
The structure of human fascin has been determined to a resolution of 1.8 Å (PDBID 3LLP) and reveals an arrangement of four tandem beta-trefoil domains that form a two lobed structure with pseudo 2-fold symmetry. It is stabilized by a hydrophobic core and a hydrophilic surface since it is often found inside cell cytoplasm in the formation of filopodia. [1]
References
- ^ a b PDB: 1DFC; Sedeh RS, Fedorov AA, Fedorov EV, Ono S, Matsumura F, Almo SC, Bathe M (July 2010). "Structure, evolutionary conservation, and conformational dynamics of Homo sapiens fascin-1, an F-actin crosslinking protein". J. Mol. Biol. 400 (3): 589–604. doi: 10.1016/j.jmb.2010.04.043. PMC 7141155. PMID 20434460.
- ^ Bryan J, Edwards R, Matsudaira P, Otto J, Wulfkuhle J (October 1993). "Fascin, an echinoid actin-bundling protein, is a homolog of the Drosophila singed gene product". Proceedings of the National Academy of Sciences of the United States of America. 90 (19): 9115–9119. Bibcode: 1993PNAS...90.9115B. doi: 10.1073/pnas.90.19.9115. ISSN 0027-8424. PMC 47512. PMID 8415664.
- ^ Edwards RA, Bryan J (1995). "Fascins, a family of actin bundling proteins". Cell Motility and the Cytoskeleton. 32 (1). John Wiley & Sons: 1–9. doi: 10.1002/cm.970320102. ISSN 0886-1544. PMID 8674129.
- ^ Yamashiro-Matsumura S, Matsumura F (April 1985). "Purification and characterization of an F-actin-bundling 55-kilodalton protein from HeLa cells". Journal of Biological Chemistry. 260 (8): 5087–5097. doi: 10.1016/S0021-9258(18)89183-9. ISSN 0021-9258. PMID 3886649.
- ^ Bryan J, Kane RE (October 1978). "Separation and interaction of the major components of sea urchin actin gel". Journal of Molecular Biology. 125 (2): 207–224. doi: 10.1016/0022-2836(78)90345-5. ISSN 0022-2836. PMID 731692.
- ^ a b Adams JC (October 2004). "Roles of fascin in cell adhesion and motility". Current Opinion in Cell Biology. 16 (5): 590–596. doi: 10.1016/j.ceb.2004.07.009. ISSN 0955-0674. PMID 15363811.
- ^ a b Hashimoto Y, Skacel M, Adams JC (September 2005). "Roles of fascin in human carcinoma motility and signaling: Prospects for a novel biomarker?". The International Journal of Biochemistry & Cell Biology. 37 (9). Elsevier: 1787–1804. doi: 10.1016/j.biocel.2005.05.004. ISSN 1357-2725. PMID 16002322.
- ^ Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD (September 1996). "beta-Catenin associates with the actin-bundling protein fascin in a noncadherin complex". Journal of Cell Biology. 134 (5). Rockefeller University Press: 1271–1281. doi: 10.1083/jcb.134.5.1271. ISSN 0021-9525. PMC 2120989. PMID 8794867.
- ^ Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM (Feb 2010). "The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion". Curr. Biol. 20 (4): 339–45. Bibcode: 2010CBio...20..339L. doi: 10.1016/j.cub.2009.12.035. PMC 3163294. PMID 20137952.
- ^ Chen J, Ganguly A, Mucsi AD, Meng J, Yan J, Detampel P, Munro F, Zhang Z, Wu M, Hari A, Stenner MD, Zheng W, Kubes P, Xia T, Amrein MW, Qi H, Shi Y (2017-01-12). "Strong adhesion by regulatory T cells induces dendritic cell cytoskeletal polarization and contact-dependent lethargy". Journal of Experimental Medicine. 214 (2): –20160620. doi: 10.1084/jem.20160620. eISSN 1540-9538. ISSN 0022-1007. PMC 5294852. PMID 28082358.
- ^ Vlahopoulos SA, Cen O, Hengen N, Agan J, Moschovi M, Critselis E, Adamaki M, Bacopoulou F, Copland JA, Boldogh I, Karin M, Chrousos GP (20 June 2015). "Dynamic aberrant NF-κB spurs tumorigenesis: A new model encompassing the microenvironment". Cytokine & Growth Factor Reviews. 26 (4): 389–403. doi: 10.1016/j.cytogfr.2015.06.001. PMC 4526340. PMID 26119834.
- ^ Grothey A, Hashizume R, Sahin AA, McCrea PD (2000). "Fascin, an actin-bundling protein associated with cell motility, is upregulated in hormone receptor negative breast cancer". British Journal of Cancer. 83 (7): 870–873. doi: 10.1054/bjoc.2000.1395. ISSN 0007-0920. PMC 2374674. PMID 10970687.
- ^ Jawhari AU, Buda A, Jenkins M, Shehzad K, Sarraf C, Noda M, Farthing MJ, Pignatelli M, Adams JC (January 2003). "Fascin, an actin-bundling protein, modulates colonic epithelial cell invasiveness and differentiation in vitro". American Journal of Pathology. 162 (1). American Society for Investigative Pathology: 69–80. doi: 10.1016/S0002-9440(10)63799-6. ISSN 0002-9440. PMC 1851132. PMID 12507891.
- ^ Vignjevic D, Schoumacher M, Gavert N, Janssen KP, Jih G, Laé M, Louvard D, Ben-Ze'ev A, Robine S (July 2007). "Fascin, a Novel Target of β-Catenin-TCF Signaling, Is Expressed at the Invasive Front of Human Colon Cancer". Cancer Research. 67 (14): 6844–6853. doi: 10.1158/0008-5472.CAN-07-0929. ISSN 0008-5472. PMID 17638895.
- ^ Hashimoto Y, Ito T, Inoue H, Okumura T, Tanaka E, Tsunoda S, Higashiyama M, Watanabe G, Imamura M, Shimada Y (April 2005). "Prognostic Significance of Fascin Overexpression in Human Esophageal Squamous Cell Carcinoma". Clinical Cancer Research. 11 (7). American Association for Cancer Research: 2597–2605. doi: 10.1158/1078-0432.CCR-04-1378. ISSN 1078-0432. PMID 15814639.
- ^ Roh YH, Kim YH, Choi HJ, Lee KE, Roh MS (March 2009). "Fascin overexpression correlates with positive thrombospondin-1 and syndecan-1 expressions and a more aggressive clinical course in patients with gallbladder cancer". Journal of Hepato-Biliary-Pancreatic Surgery. 16 (3). Springer International: 315–21. doi: 10.1007/s00534-009-0046-1. ISSN 0944-1166. PMID 19259612.
- ^ Li A, Morton JP, Ma Y, Karim SA, Zhou Y, Faller WJ, Woodham EF, Morris HT, Stevenson RP, Juin A, Jamieson NB, MacKay CJ, Carter CR, Leung HY, Yamashiro S, Blyth K, Sansom OJ, Machesky LM (2014). "Fascin Is Regulated by Slug, Promotes Progression of Pancreatic Cancer in Mice, and Is Associated With Patient Outcomes". Gastroenterology. 146 (5): 1386–1396.e17. doi: 10.1053/j.gastro.2014.01.046. ISSN 0016-5085. PMC 4000441. PMID 24462734.
- ^ Darnel AD, Behmoaram E, Vollmer RT, Corcos J, Bijian K, Sircar K, Su J, Jiao J, Alaoui-Jamali MA, Bismar TA (February 2009). "Fascin regulates prostate cancer cell invasion and is associated with metastasis and biochemical failure in prostate cancer". Clinical Cancer Research. 15 (4). American Association for Cancer Research: 1376–1383. doi: 10.1158/1078-0432.CCR-08-1789. ISSN 1078-0432. PMID 19228738.
- ^ Jayo A, Parsons M (October 2010). "Fascin: a key regulator of cytoskeletal dynamics". Int. J. Biochem. Cell Biol. 42 (10): 1614–7. doi: 10.1016/j.biocel.2010.06.019. PMID 20601080.
External links
- fascin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)