 | |
| Clinical data | |
|---|---|
| Trade names | Zepsun |
| Other names | CM-4307 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
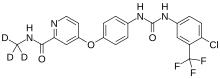
| Formula | C21H16ClF3N4O3 |
| Molar mass | 464.83 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Donafenib, sold under the brand name Zepsun, is a pharmaceutical drug for the treatment of cancer.
In China, donafenib is approved for the treatment of unresectable hepatocellular carcinoma in patients who have not previously received systemic treatment. [1] [2]
Donafenib is a kinase inhibitor that targets Raf kinase and various receptor tyrosine kinases. [3] It is a deuterated derivative of sorafenib with improved pharmacokinetic properties. [4] [5]
References
- ^ Keam SJ, Duggan S (November 2021). "Donafenib: First Approval". Drugs. 81 (16): 1915–1920. doi: 10.1007/s40265-021-01603-0. PMID 34591285.
- ^ Chen R, Ielasi L, di Carlo A, Tovoli F (February 2023). "Donafenib in hepatocellular carcinoma". Drugs of Today. 59 (2): 83–90. doi: 10.1358/dot.2023.59.2.3507751. PMID 36811408.
- ^ "Donafenib". NCI Cancer Dictionary. National Cancer Institute, National Institutes of Health.
- ^ Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. (September 2021). "Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial". Journal of Clinical Oncology. 39 (27): 3002–3011. doi: 10.1200/JCO.21.00163. PMC 8445562. PMID 34185551.
- ^ Qin S, Bi F, Xu J, Du C, Fan Q, Zhang L, et al. (2020). "P-86 Comparison of the pharmacokinetics of donafenib and sorafenib in patients with advanced hepatocellular carcinoma: An open-label, randomized, parallel-controlled, multicentre phase II/III trial". Annals of Oncology. 31: S117–S118. doi: 10.1016/j.annonc.2020.04.168.
 | |
| Clinical data | |
|---|---|
| Trade names | Zepsun |
| Other names | CM-4307 |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard ( EPA) | |
| Chemical and physical data | |
| Formula | C21H16ClF3N4O3 |
| Molar mass | 464.83 g·mol−1 |
| 3D model ( JSmol) | |
| |
| |
Donafenib, sold under the brand name Zepsun, is a pharmaceutical drug for the treatment of cancer.
In China, donafenib is approved for the treatment of unresectable hepatocellular carcinoma in patients who have not previously received systemic treatment. [1] [2]
Donafenib is a kinase inhibitor that targets Raf kinase and various receptor tyrosine kinases. [3] It is a deuterated derivative of sorafenib with improved pharmacokinetic properties. [4] [5]
References
- ^ Keam SJ, Duggan S (November 2021). "Donafenib: First Approval". Drugs. 81 (16): 1915–1920. doi: 10.1007/s40265-021-01603-0. PMID 34591285.
- ^ Chen R, Ielasi L, di Carlo A, Tovoli F (February 2023). "Donafenib in hepatocellular carcinoma". Drugs of Today. 59 (2): 83–90. doi: 10.1358/dot.2023.59.2.3507751. PMID 36811408.
- ^ "Donafenib". NCI Cancer Dictionary. National Cancer Institute, National Institutes of Health.
- ^ Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. (September 2021). "Donafenib Versus Sorafenib in First-Line Treatment of Unresectable or Metastatic Hepatocellular Carcinoma: A Randomized, Open-Label, Parallel-Controlled Phase II-III Trial". Journal of Clinical Oncology. 39 (27): 3002–3011. doi: 10.1200/JCO.21.00163. PMC 8445562. PMID 34185551.
- ^ Qin S, Bi F, Xu J, Du C, Fan Q, Zhang L, et al. (2020). "P-86 Comparison of the pharmacokinetics of donafenib and sorafenib in patients with advanced hepatocellular carcinoma: An open-label, randomized, parallel-controlled, multicentre phase II/III trial". Annals of Oncology. 31: S117–S118. doi: 10.1016/j.annonc.2020.04.168.