A major contributor to this article appears to have a
close connection with its subject. (August 2019) |
Digital polymerase chain reaction (digital PCR, DigitalPCR, dPCR, or dePCR) is a biotechnological refinement of conventional polymerase chain reaction methods that can be used to directly quantify and clonally amplify nucleic acids strands including DNA, cDNA, or RNA. The key difference between dPCR and qPCR lies in the method of measuring nucleic acids amounts, with the former being a more precise method than PCR, though also more prone to error in the hands of inexperienced users. [1] PCR carries out one reaction per single sample. dPCR also carries out a single reaction within a sample, however the sample is separated into a large number of partitions and the reaction is carried out in each partition individually. This separation allows a more reliable collection and sensitive measurement of nucleic acid amounts. The method has been demonstrated as useful for studying variations in gene sequences — such as copy number variants and point mutations.

The polymerase chain reaction method is used to quantify nucleic acids by amplifying a nucleic acid molecule with the enzyme DNA polymerase. [2] Conventional PCR is based on the theory that amplification is exponential. Therefore, nucleic acids may be quantified by comparing the number of amplification cycles and amount of PCR end-product to those of a reference sample. [3] However, many factors complicate this calculation, creating uncertainties and inaccuracies. These factors include the following: initial amplification cycles may not be exponential; PCR amplification eventually plateaus after an uncertain number of cycles; and low initial concentrations of target nucleic acid molecules may not amplify to detectable levels. However, the most significant limitation of PCR is that PCR amplification efficiency in a sample of interest may be different from that of reference samples.

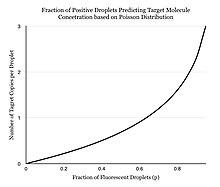
Instead of performing one reaction per well, dPCR involves partitioning the PCR solution into tens of thousands of nano-liter sized droplets, where a separate PCR reaction takes place in each one. [4] [5] A PCR solution is made similarly to a TaqMan assay, which consists of template DNA (or RNA), fluorescence-quencher probes, primers, and a PCR master mix, which contains DNA polymerase, dNTPs, MgCl2, and reaction buffers at optimal concentrations. Several different methods can be used to partition samples, including microwell plates, capillaries, oil emulsion, and arrays of miniaturized chambers with nucleic acid binding surfaces. [6] The PCR solution is partitioned into smaller units, each with the necessary components for amplification. The partitioned units are then subjected to thermocycling so that each unit may independently undergo PCR amplification. After multiple PCR amplification cycles, the samples are checked for fluorescence with a binary readout of “0” or “1”. The fraction of fluorescing droplets is recorded. [5] The partitioning of the sample allows one to estimate the number of different molecules by assuming that the molecule population follows the Poisson distribution, thus accounting for the possibility of multiple target molecules inhabiting a single droplet. Using Poisson's law of small numbers, the distribution of target molecule within the sample can be accurately approximated allowing for a quantification of the target strand in the PCR product. [7] This model simply predicts that as the number of samples containing at least one target molecule increases, the probability of the samples containing more than one target molecule increases. [8] In conventional PCR, the number of PCR amplification cycles is proportional to the starting copy number. Different from many people's belief that dPCR provides absolute quantification, digital PCR uses statistical power to provide relative quantification. For example, if Sample A, when assayed in 1 million partitions, gives one positive reaction, it does not mean that the Sample A has one starting molecule.[ citation needed]
The benefits of dPCR include increased precision through massive sample partitioning, which ensures reliable measurements in the desired DNA sequence due to reproducibility. [5] Error rates are larger when detecting small-fold change differences with basic PCR, while error rates are smaller with dPCR due to the smaller-fold change differences that can be detected in DNA sequence. The technique itself reduces the use of a larger volume of reagent needed, which inevitably will lower experiment cost. Also, dPCR is highly quantitative as it does not rely on relative fluorescence of the solution to determine the amount of amplified target DNA.
dPCR measures the actual number of molecules (target DNA) as each molecule is in one droplet, thus making it a discrete “digital” measurement. It provides absolute quantification because dPCR measures the positive fraction of samples, which is the number of droplets that are fluorescing due to proper amplification. This positive fraction accurately indicates the initial amount of template nucleic acid. Similarly, qPCR utilizes fluorescence; however, it measures the intensity of fluorescence at specific times (generally after every amplification cycle) to determine the relative amount of target molecule (DNA), but cannot specify the exact amount without constructing a standard curve using different amounts of a defined standard. It gives the threshold per cycle (CT) and the difference in CT is used to calculate the amount of initial nucleic acid. As such, qPCR is an analog measurement, which may not be as precise due to the extrapolation required to attain a measurement. [6] [9]
dPCR measures the amount of DNA after amplification is complete and then determines the fraction of replicates. This is representative of an endpoint measurement as it requires the observation of the data after the experiment is completed. In contrast, qPCR records the relative fluorescence of the DNA at specific points during the amplification process, which requires stops in the experimental process. This “real-time” aspect of qPCR may theoretically affect results due to the stopping of the experiment.[ citation needed] In practice, however, most qPCR thermal cyclers read each sample's fluorescence very quickly at the end of the annealing/extension step before proceeding to the next melting step, meaning this hypothetical concern is not actually relevant or applicable for the vast majority of researchers. dPCR measures the amplification by measuring the products of end point PCR cycling and is therefore less susceptible to the artifacts arising from impaired amplification efficiencies due to the presence of PCR inhibitors or primer template mismatch. [10] [11]
Real-time Digital PCR (rdPCR) combines the methodologies of digital PCR (dPCR) and quantitative PCR (qPCR), integrating the precision of dPCR with the real-time analysis capabilities of qPCR. This integration aims to provide enhanced sensitivity, specificity, and the ability for absolute quantification of nucleic acid sequences, contributing to the quantification of genetic material in scientific and clinical research. [12] [13]
qPCR is unable to distinguish differences in gene expression or copy number variations that are smaller than twofold. On the other hand, dPCR has a higher precision and has been shown to detect differences of less than 30% in gene expression, distinguish between copy number variations that differ by only 1 copy, and identify alleles that occur at frequencies less than 0.1%. [14] [5]
Digital PCR has many applications in basic research, clinical diagnostics and environmental testing. Its uses include pathogen detection and digestive health analysis; [15] [16] liquid biopsy for cancer monitoring, organ transplant rejection monitoring and non-invasive prenatal testing for serious genetic abnormalities; [17] [18] [19] [20] [21] [22] [23] [24] copy number variation analysis, [25] [26] [27] single gene expression analysis, [28] rare sequence detection, [24] [29] [30] gene expression profiling and single-cell analysis; [31] [32] [30] [33] [34] [35] [36] the detection of DNA contaminants in bioprocessing, [37] the validation of gene edits and detection of specific methylation changes in DNA as biomarkers of cancer. [38] [39] [40] dPCR is also frequently used as an orthogonal method to confirm rare mutations detected through next-generation sequencing (NGS) and to validate NGS libraries. [41] [42] [43]
dPCR enables the absolute and reproducible quantification of target nucleic acids at single-molecule resolution. [30] [44] [45] [46] Unlike analogue quantitative PCR (qPCR), however, absolute quantification with dPCR does not require a standard curve. [44] dPCR also has a greater tolerance for inhibitor substances and PCR assays that amplify inefficiently as compared to qPCR. [47] [11]
dPCR can quantify, for example, the presence of specific sequences from contaminating genetically modified organisms in foodstuffs, [48] viral load in the blood, [49] PBMCs, [50] [51] serum samples, [52] chorionic villi tissues, [50] [51] biomarkers of neurodegenerative disease in cerebral spinal fluid, [53] and fecal contamination in drinking water. [54]
An alteration in copy number state with respect to a single-copy reference locus is referred to as a “ copy number variation” (CNV) if it appears in germline cells, or a copy number alteration (CNA) if it appears in somatic cells. [55] A CNV or CNA could be due to a deletion or amplification of a locus with respect to the number of copies of the reference locus present in the cell, and together, they are major contributors to variability in the human genome. [56] [57] [58] They have been associated with cancers; [59] [60] [61] neurological, [62] psychiatric, [63] [64] and autoimmune diseases; [65] and adverse drug reactions. [66] However, it is difficult to measure these allelic variations with high precision using other methods such as qPCR, thus making phenotypic and disease associations with altered CNV status challenging. [67] [68]
The large number of “digitized,” endpoint measurements made possible by sample partitioning enables dPCR to resolve small differences in copy number with better accuracy and precision when compared to other methods such as SNP-based microarrays [69] or qPCR. [70] [71] qPCR is limited in its ability to precisely quantify gene amplifications in several diseases, including Crohn’s disease, HIV-1 infection, and obesity. [72] [68] [71]
dPCR was designed to measure the concentration of a nucleic acid target in copies per unit volume of the sample. When operating in dilute reactions where less than ~10% of the partitions contain a desired target (referred to as “limiting dilution”), copy number can be estimated by comparing the number of fluorescent droplets arising from a target CNV with the number of fluorescent droplets arising from an invariant single-copy reference locus. [25] In fact, both at these lower target concentrations and at higher ones where multiple copies of the same target can co-localize to a single partition, Poisson statistics are used to correct for these multiple occupancies to give a more accurate value for each target’s concentration. [73] [6]
Digital PCR has been used to uncover both germline and somatic variation in gene copy number between humans [74] and to study the link between amplification of HER2 (ERBB2) and breast cancer progression. [75] [76] [77] [27]
Partitioning in digital PCR increases sensitivity and allows for detection of rare events, especially single nucleotide variants (SNVs), by isolating or greatly diminishing the target biomarker signal from potentially competing background. [9] [6] These events can be organized into two classes: rare mutation detection and rare sequence detection.
Rare mutation detection occurs when a biomarker exists within a background of a highly abundant counterpart that differs by only a single nucleotide variant (SNV). Digital PCR has been shown to be capable of detecting mutant DNA in the presence of a 200,000-fold excess of wild type background, which is 2,000 times more sensitive than achievable with conventional qPCR. [9]
Digital PCR can detect rare sequences such as HIV DNA in patients with HIV, [24] and DNA from fecal bacteria in ocean and other water samples for assessing water quality. [78] dPCR can detect sequences as rare as 1 in every 1,250,000 cells. [24]
dPCR’s ability to detect rare mutations may be of particular benefit in the clinic through the use of the liquid biopsy, a generally noninvasive strategy for detecting and monitoring disease via bodily fluids. [17] [79] Researchers have used liquid biopsy to monitor tumor load, treatment response and disease progression in cancer patients by measuring rare mutations in circulating tumor DNA (ctDNA) in a variety of biological fluids from patients including blood, urine and cerebrospinal fluid. [17] [80] [81] Early detection of ctDNA (as in molecular relapse) may lead to earlier administration of an immunotherapy or a targeted therapy specific for the patient’s mutation signature, potentially improving chances of the treatment’s effectiveness rather than waiting for clinical relapse before altering treatment. Liquid biopsies can have turnaround times of a few days, compared to two to four weeks or longer for tissue-based tests. [82] [83] This reduced time to results has been used by physicians to expedite treatments tailored to biopsy data. [82]
In 2016, a prospective trial using dPCR at the Dana-Farber Cancer Institute authenticated the clinical benefit of liquid biopsy as a predictive diagnostic tool for patients with non-small-cell lung cancer. [84] The application of liquid biopsy tests have also been studied in patients with breast, [85] colorectal, [86] [87] gynecologic, [88] and bladder cancers [80] [89] to monitor both the disease load and the tumor’s response to treatment.
Gene expression and RNA quantification studies have benefited from the increased precision and absolute quantification of dPCR. [90] RNA quantification can be accomplished via RT-PCR, wherein RNA is reverse-transcribed into cDNA in the partitioned reaction itself, and the number of RNA molecules originating from each transcript (or allelic transcript) is quantified via dPCR. [31]
One can often achieve greater sensitivity and precision by using dPCR rather than qPCR to quantify RNA molecules in part because it does not require use of a standard curve for quantification. [91] dPCR is also more resilient to PCR inhibitors for the quantification of RNA than qPCR. [47] [16] [90]
dPCR can detect and quantify more individual target species per detection channel than qPCR by virtue of being able to distinguish targets based on their differential fluorescence amplitude or by the use of distinctive color combinations for their detection. [92] [90] As an example of this, a 2-channel dPCR system has been used to detect in a single well the expression of four different splice variants of human telomerase reverse transcriptase, a protein that is more active in most tumor cells than in healthy cells. [93]
Using the dynamic partitioning capabilities employed in dPCR, improved NGS sequencing can be achieved by partitioning of complex PCR reactions prior to amplification to give more uniform amplification across many distinct amplicons for NGS analysis. [94] [95] Additionally, the improved specificity of complex PCR amplification reactions in droplets has been shown to greatly reduce the number of iterations required to select for high affinity aptamers in the SELEX method. [96] Partitioning can also allow for more robust measurements of telomerase activity from cell lysates. [97] [98] dPCR’s dynamic partitioning capabilities can also be used to partition thousands of nuclei or whole cells into individual droplets to facilitate library preparation for a single cell assay for transposase-accessible chromatin using sequencing (scATAC-seq). [99]
Droplet Digital PCR (ddPCR) is a method of dPCR in which a 20 microliter sample reaction including assay primers and either Taqman probes or an intercalating dye, is divided into ~20,000 nanoliter-sized oil droplets through a water-oil emulsion technique, thermocycled to endpoint in a 96-well PCR plate, and fluorescence amplitude read for all droplets in each sample well in a droplet flow cytometer. [100]
Chip-based Digital PCR (dPCR) is also a method of dPCR in which the reaction mix (also when used in qPCR) is divided into ~10,000 to ~45,000 partitions on a chip, then amplified using an endpoint PCR thermocycling machine, and is read using a high-powered camera reader with fluorescence filter (HEX, FAM, Cy5, Cy5.5 and Texas Red) for all partitions on each chip. [101]
dPCR rose out of an approach first published in 1988 by Cetus Corporation when researchers showed that a single copy of the β-globin gene could be detected and amplified by PCR. [102] [103] This was achieved by diluting DNA samples from a normal human cell line with DNA from a mutant line having a homozygous deletion of the β-globin gene, until it was no longer present in the reaction. In 1989, Peter Simmonds, AJ Brown et al. used this concept to quantify a molecule for the first time. [104] Alex Morley and Pamela Sykes formally established the method as a quantitative technique in 1992. [45]
In 1999, Bert Vogelstein and Kenneth Kinzler coined the term “digital PCR” and showed that the technique could be used to find rare cancer mutations. [105] However, dPCR was difficult to perform; it was labor intensive, required a lot of training to do properly, and was difficult to do in large quantities. [105] In 2003, Kinzler and Vogelstein continued to refine dPCR and created an improved method that they called BEAMing technology, an acronym for “beads, emulsion, amplification and magnetics.” The new protocol used emulsion to compartmentalize amplification reactions in a single tube. This change made it possible for scientists to scale the method to thousands of reactions in a single run. [106] [107] [108]
Companies developing commercial dPCR systems have integrated technologies like automated partitioning of samples, digital counting of nucleic acid targets, and increasing droplet count that can help the process be more efficient. [109] [110] [111] In recent years, scientists have developed and commercialized dPCR-based diagnostics for several conditions, including non-small cell lung cancer and Down’s Syndrome. [112] [113] The first dPCR system for clinical use was CE-marked in 2017 and cleared by the US Food and Drug Administration in 2019, for diagnosing chronic myeloid leukemia. [114]
- ^ Perkel J (May 2015). "Guiding our PCR experiments". BioTechniques. 58 (5): 217–221. doi: 10.2144/000114283. PMID 25967899.
- ^ "Polymerase Chain Reaction (PCR)". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Higuchi, Russell; Fockler, Carita; Dollinger, Gavin; Watson, Robert (September 1993). "Kinetic PCR Analysis: Real-time Monitoring of DNA Amplification Reactions". Bio/Technology. 11 (9): 1026–1030. doi: 10.1038/nbt0993-1026. ISSN 1546-1696. PMID 7764001. S2CID 5714001.
- ^ Duewer DL, Kline MC, Romsos EL, Toman B (May 2018). "Evaluating droplet digital PCR for the quantification of human genomic DNA: converting copies per nanoliter to nanograms nuclear DNA per microliter". Analytical and Bioanalytical Chemistry. 410 (12): 2879–2887. doi: 10.1007/s00216-018-0982-1. PMC 5996397. PMID 29556737.
- ^ a b c d Baker M (2012). "Digital PCR hits its stride". Nature Methods. 9 (6): 541–544. doi: 10.1038/nmeth.2027. S2CID 46347563.
- ^ a b c d Quan PL, Sauzade M, Brouzes E (April 2018). "dPCR: A Technology Review". Sensors. 18 (4): 1271. Bibcode: 2018Senso..18.1271Q. doi: 10.3390/s18041271. PMC 5948698. PMID 29677144.
- ^ Prediger E. "Digital PCR (dPCR)—What is it and why use it?". Integrated DNA Technologies.
- ^ Butler DM, Pacold ME, Jordan PS, Richman DD, Smith DM (December 2009). "The efficiency of single genome amplification and sequencing is improved by quantitation and use of a bioinformatics tool". Journal of Virological Methods. 162 (1–2): 280–283. doi: 10.1016/j.jviromet.2009.08.002. PMC 2761514. PMID 19698751.
- ^ a b c Pekin D, Skhiri Y, Baret JC, Le Corre D, Mazutis L, Salem CB, et al. (July 2011). "Quantitative and sensitive detection of rare mutations using droplet-based microfluidics". Lab on a Chip. 11 (13): 2156–2166. doi: 10.1039/c1lc20128j. PMID 21594292.
- ^ Svec D, Tichopad A, Novosadova V, Pfaffl MW, Kubista M (March 2015). "How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments". Biomolecular Detection and Quantification. 3: 9–16. doi: 10.1016/j.bdq.2015.01.005. PMC 4822216. PMID 27077029.
- ^ a b Dingle TC, Sedlak RH, Cook L, Jerome KR (November 2013). "Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances". Clinical Chemistry. 59 (11): 1670–1672. doi: 10.1373/clinchem.2013.211045. PMC 4247175. PMID 24003063.
- ^ Pavšič, Jernej; Žel, Jana; Milavec, Mojca (2016-01-01). "Assessment of the real-time PCR and different digital PCR platforms for DNA quantification". Analytical and Bioanalytical Chemistry. 408 (1): 107–121. doi: 10.1007/s00216-015-9107-2. ISSN 1618-2650. PMC 4706846. PMID 26521179.
- ^ Xu, Jiachen; Duong, Kyra; Yang, Zhenlin; Kaji, Kavanaugh; Ou, Jiajia; Head, Steven R.; Crynen, Gogce; Ordoukhanian, Phillip; Hanna, Lauren; Hanna, Ava; Wang, Yan; Wang, Zhijie; Wang, Jie (December 2021). "Real-time digital polymerase chain reaction (PCR) as a novel technology improves limit of detection for rare allele assays". Translational Lung Cancer Research. 10 (12): 4336–4352. doi: 10.21037/tlcr-21-728. ISSN 2226-4477. PMC 8743530. PMID 35070745.
- ^ Salipante SJ, Jerome KR (January 2020). "Digital PCR-An Emerging Technology with Broad Applications in Microbiology". Clinical Chemistry. 66 (1): 117–123. doi: 10.1373/clinchem.2019.304048. PMID 31704712.
- ^ Witte AK, Fister S, Mester P, Schoder D, Rossmanith P (November 2016). "Evaluation of the performance of quantitative detection of the Listeria monocytogenes prfA locus with droplet digital PCR". Analytical and Bioanalytical Chemistry. 408 (27): 7583–7593. doi: 10.1007/s00216-016-9861-9. PMC 5061835. PMID 27558101.
- ^ a b Stauber J, Shaikh N, Ordiz MI, Tarr PI, Manary MJ (May 2016). "Droplet digital PCR quantifies host inflammatory transcripts in feces reliably and reproducibly". Cellular Immunology. 303: 43–49. doi: 10.1016/j.cellimm.2016.03.007. PMC 4863679. PMID 27063479.
- ^ a b c Skibo S (23 Feb 2018). "Has Tumor Profiling Caught Up to Cancer?". Retrieved 23 July 2019.
- ^ Hirsch F (27 July 2018). "Guidelines highlight 'best practices' for liquid biopsy during treatment of non-small cell lung cancer". Retrieved 23 July 2019.
- ^ Johnson M (12 Jan 2018). "Bio-Rad Continues to Advance Digital PCR Tech, Liquid Biopsy Tests Into Commercial Clinical Market". Retrieved 23 July 2019.
- ^ Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O'Connell A, Messineo MM, et al. (March 2014). "Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA". Clinical Cancer Research. 20 (6): 1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. PMC 3959249. PMID 24429876.
- ^ Schütz E, Fischer A, Beck J, Harden M, Koch M, Wuensch T, et al. (April 2017). "Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study". PLOS Medicine. 14 (4): e1002286. doi: 10.1371/journal.pmed.1002286. PMC 5404754. PMID 28441386.
- ^ Lee SY, Hwang SY (2015). "Application of digital polymerase chain reaction technology for noninvasive prenatal test". Journal of Genetic Medicine. 12 (2): 72–78. doi: 10.5734/JGM.2015.12.2.72. ISSN 2383-8442.
- ^ Gu W, Koh W, Blumenfeld YJ, El-Sayed YY, Hudgins L, Hintz SR, Quake SR (July 2014). "Noninvasive prenatal diagnosis in a fetus at risk for methylmalonic acidemia". Genetics in Medicine. 16 (7): 564–567. doi: 10.1038/gim.2013.194. PMC 4079742. PMID 24406457.
- ^ a b c d Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, et al. (2013). "Highly precise measurement of HIV DNA by droplet digital PCR". PLOS ONE. 8 (4): e55943. Bibcode: 2013PLoSO...855943S. doi: 10.1371/journal.pone.0055943. PMC 3616050. PMID 23573183.
- ^
a
b Bell AD, Usher CL, McCarroll SA (2018). "Analyzing Copy Number Variation with Droplet Digital PCR". Digital PCR. Methods in Molecular Biology. Vol. 1768. Clifton, N.J. pp. 143–160.
doi:
10.1007/978-1-4939-7778-9_9.
ISBN
978-1-4939-7776-5.
PMID
29717442.
{{ cite book}}: CS1 maint: location missing publisher ( link) - ^ Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J, et al. (January 2017). "Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer". Gastric Cancer. 20 (1): 126–135. doi: 10.1007/s10120-016-0599-z. PMID 26874951.
- ^ a b Gevensleben H, Garcia-Murillas I, Graeser MK, Schiavon G, Osin P, Parton M, et al. (June 2013). "Noninvasive detection of HER2 amplification with plasma DNA digital PCR". Clinical Cancer Research. 19 (12): 3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. PMC 6485473. PMID 23637122.
- ^ Mazzoni E, Frontini F, Rotondo JC, Zanotta N, Fioravanti A, Minelli F, et al. (April 2019). "Antibodies reacting to mimotopes of Simian virus 40 large T antigen, the viral oncoprotein, in sera from children". Journal of Cellular Physiology. 234 (4): 3170–3179. doi: 10.1002/jcp.27490. hdl: 11392/2397717. PMID 30362540. S2CID 53106591.
- ^ Uchiyama Y, Nakashima M, Watanabe S, Miyajima M, Taguri M, Miyatake S, et al. (March 2016). "Ultra-sensitive droplet digital PCR for detecting a low-prevalence somatic GNAQ mutation in Sturge-Weber syndrome". Scientific Reports. 6 (1): 22985. Bibcode: 2016NatSR...622985U. doi: 10.1038/srep22985. PMC 4783707. PMID 26957145.
- ^ a b c Marusina K (1 Oct 2017). "Positioning Digital PCR for Sharper Genomic Views". Retrieved 23 July 2019.
- ^ a b Kamitaki N, Usher CL, McCarroll SA (2018). "Using Droplet Digital PCR to Analyze Allele-Specific RNA Expression". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 401–422. doi: 10.1007/978-1-4939-7778-9_23. ISBN 978-1-4939-7776-5. PMID 29717456.
- ^ Millier MJ, Stamp LK, Hessian PA (December 2017). "Digital-PCR for gene expression: impact from inherent tissue RNA degradation". Scientific Reports. 7 (1): 17235. Bibcode: 2017NatSR...717235M. doi: 10.1038/s41598-017-17619-0. PMC 5722939. PMID 29222437.
- ^ "Highly Sensitive Detection of Hepatitis B Using ddPCR". 12 Apr 2018. Retrieved 23 July 2019.
- ^ Jang M, Jeong SW, Bae NH, Song Y, Lee TJ, Lee MK, Lee SJ, Lee KG (2017). "Droplet-based digital PCR system for detection of single-cell level of foodborne pathogens". BioChip Journal. 11 (4): 329–337. doi: 10.1007/s13206-017-1410-x. ISSN 2092-7843. S2CID 89829687.
- ^ Igarashi Y, Uchiyama T, Minegishi T, Takahashi S, Watanabe N, Kawai T, et al. (September 2017). "Single Cell-Based Vector Tracing in Patients with ADA-SCID Treated with Stem Cell Gene Therapy". Molecular Therapy. Methods & Clinical Development. 6: 8–16. doi: 10.1016/j.omtm.2017.05.005. PMC 5466583. PMID 28626778.
- ^ Albayrak C, Jordi CA, Zechner C, Lin J, Bichsel CA, Khammash M, Tay S (March 2016). "Digital Quantification of Proteins and mRNA in Single Mammalian Cells". Molecular Cell. 61 (6): 914–924. doi: 10.1016/j.molcel.2016.02.030. PMID 26990994.
- ^ Hussain M, Fantuzzo R, Mercorelli S, Cullen C (May 2016). "A direct droplet digital PCR method for quantification of residual DNA in protein drugs produced in yeast cells". Journal of Pharmaceutical and Biomedical Analysis. 123: 128–131. doi: 10.1016/j.jpba.2016.01.050. PMID 26896631.
- ^ Miyaoka Y, Chan AH, Judge LM, Yoo J, Huang M, Nguyen TD, et al. (March 2014). "Isolation of single-base genome-edited human iPS cells without antibiotic selection". Nature Methods. 11 (3): 291–293. doi: 10.1038/nmeth.2840. PMC 4063274. PMID 24509632.
- ^ Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, et al. (January 2016). "In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy". Science. 351 (6271): 403–407. Bibcode: 2016Sci...351..403N. doi: 10.1126/science.aad5143. PMC 4883596. PMID 26721684.
- ^ Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, et al. (March 2016). "Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing". Scientific Reports. 6: 23549. Bibcode: 2016NatSR...623549M. doi: 10.1038/srep23549. PMC 4814844. PMID 27030102.
- ^ Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, et al. (July 2015). "Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer". Clinical Chemistry. 61 (7): 974–982. doi: 10.1373/clinchem.2015.238717. PMID 25979954.
- ^ Robin JD, Ludlow AT, LaRanger R, Wright WE, Shay JW (April 2016). "Comparison of DNA Quantification Methods for Next Generation Sequencing". Scientific Reports. 6 (1): 24067. Bibcode: 2016NatSR...624067R. doi: 10.1038/srep24067. PMC 4822169. PMID 27048884.
- ^ Aigrain L, Gu Y, Quail MA (June 2016). "Quantitation of next generation sequencing library preparation protocol efficiencies using droplet digital PCR assays - a systematic comparison of DNA library preparation kits for Illumina sequencing". BMC Genomics. 17 (1): 458. doi: 10.1186/s12864-016-2757-4. PMC 4906846. PMID 27297323.
- ^ a b Brunetto GS, Massoud R, Leibovitch EC, Caruso B, Johnson K, Ohayon J, et al. (August 2014). "Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations". Journal of Neurovirology. 20 (4): 341–351. doi: 10.1007/s13365-014-0249-3. PMC 4085507. PMID 24781526.
- ^ a b Sykes PJ, Neoh SH, Brisco MJ, Hughes E, Condon J, Morley AA (September 1992). "Quantitation of targets for PCR by use of limiting dilution". BioTechniques. 13 (3): 444–449. PMID 1389177.
- ^ Vogelstein B, Kinzler KW (August 1999). "Digital PCR". Proceedings of the National Academy of Sciences of the United States of America. 96 (16): 9236–9241. Bibcode: 1999PNAS...96.9236V. doi: 10.1073/pnas.96.16.9236. PMC 17763. PMID 10430926.
- ^ a b Rački N, Dreo T, Gutierrez-Aguirre I, Blejec A, Ravnikar M (2014). "Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples". Plant Methods. 10 (1): 42. doi: 10.1186/s13007-014-0042-6. PMC 4307183. PMID 25628753.
- ^ Dobnik D, Spilsberg B, Bogožalec Košir A, Štebih D, Morisset D, Holst-Jensen A, Žel J (2018). "Multiplex Droplet Digital PCR Protocols for Quantification of GM Maize Events". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 69–98. doi: 10.1007/978-1-4939-7778-9_5. ISBN 978-1-4939-7776-5. PMID 29717438.
- ^ Vellucci A, Leibovitch EC, Jacobson S (2018). "Using Droplet Digital PCR to Detect Coinfection of Human Herpesviruses 6A and 6B (HHV-6A and HHV-6B) in Clinical Samples". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 99–109. doi: 10.1007/978-1-4939-7778-9_6. ISBN 978-1-4939-7776-5. PMID 29717439.
- ^ a b Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Gonzalez LO, et al. (March 2020). "Droplet-digital PCR assay to detect Merkel cell polyomavirus sequences in chorionic villi from spontaneous abortion affected females". Journal of Cellular Physiology. 235 (3): 1888–1894. doi: 10.1002/jcp.29213. hdl: 11392/2409453. PMID 31549405.
- ^ a b Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M, et al. (March 2019). "Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion". Human Reproduction. 34 (3): 433–440. doi: 10.1002/jcp.27490. hdl: 11392/2397717. PMID 30590693. S2CID 53106591.
- ^ Mazzoni E, Rotondo JC, Marracino L, Selvatici R, Bononi I, Torreggiani E, et al. (2017). "Detection of Merkel Cell Polyomavirus DNA in Serum Samples of Healthy Blood Donors". Frontiers in Oncology. 7: 294. doi: 10.3389/fonc.2017.00294. PMC 5712532. PMID 29238698.
- ^ Podlesniy P, Trullas R (2018). "Biomarkers in Cerebrospinal Fluid: Analysis of Cell-Free Circulating Mitochondrial DNA by Digital PCR". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 111–126. doi: 10.1007/978-1-4939-7778-9_7. ISBN 978-1-4939-7776-5. PMID 29717440.
- ^ Cao Y, Raith MR, Griffith JF (2018). "Testing of General and Human-Associated Fecal Contamination in Waters". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 127–140. doi: 10.1007/978-1-4939-7778-9_8. ISBN 978-1-4939-7776-5. PMID 29717441.
- ^ Li W, Lee A, Gregersen PK (January 2009). "Copy-number-variation and copy-number-alteration region detection by cumulative plots". BMC Bioinformatics. 10 (S1): S67. arXiv: 0909.3129. Bibcode: 2009arXiv0909.3129L. doi: 10.1186/1471-2105-10-S1-S67. PMC 2648736. PMID 19208171.
- ^ Koren A, Handsaker RE, Kamitaki N, Karlić R, Ghosh S, Polak P, et al. (November 2014). "Genetic variation in human DNA replication timing". Cell. 159 (5): 1015–1026. doi: 10.1016/j.cell.2014.10.025. PMC 4359889. PMID 25416942.
- ^ Sanders S (16 Jul 2008). "CNVs vs SNPs: Understanding Human Structural Variation in Disease". Retrieved 24 July 2019.
- ^ Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. (January 2017). "Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects". Nature Genetics. 49 (1): 27–35. doi: 10.1038/ng.3725. PMC 5737772. PMID 27869829.
- ^ Shlien A, Malkin D (June 2009). "Copy number variations and cancer". Genome Medicine. 1 (6): 62. doi: 10.1186/gm62. PMC 2703871. PMID 19566914.
- ^ Lauer S, Gresham D (December 2019). "An evolving view of copy number variants". Current Genetics. 65 (6): 1287–1295. doi: 10.1007/s00294-019-00980-0. PMID 31076843. S2CID 149444714.
- ^ "Copy Number Alteration Found to Be Associated with Cancer Mortality". 5 Sep 2018. Retrieved 24 July 2019.
- ^ Gu W, Lupski JR (2008). "CNV and nervous system diseases--what's new?". Cytogenetic and Genome Research. 123 (1–4): 54–64. doi: 10.1159/000184692. PMC 2920183. PMID 19287139.
- ^ Thapar A, Cooper M (August 2013). "Copy number variation: what is it and what has it told us about child psychiatric disorders?". Journal of the American Academy of Child and Adolescent Psychiatry. 52 (8): 772–774. doi: 10.1016/j.jaac.2013.05.013. PMC 3919207. PMID 23880486.
- ^ Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. (February 2016). "Schizophrenia risk from complex variation of complement component 4". Nature. 530 (7589): 177–183. Bibcode: 2016Natur.530..177.. doi: 10.1038/nature16549. PMC 4752392. PMID 26814963.
- ^ Yim SH, Jung SH, Chung B, Chung YJ (May 2015). "Clinical implications of copy number variations in autoimmune disorders". The Korean Journal of Internal Medicine. 30 (3): 294–304. doi: 10.3904/kjim.2015.30.3.294. PMC 4438283. PMID 25995659.
- ^ He Y, Hoskins JM, McLeod HL (May 2011). "Copy number variants in pharmacogenetic genes". Trends in Molecular Medicine. 17 (5): 244–251. doi: 10.1016/j.molmed.2011.01.007. PMC 3092840. PMID 21388883.
- ^ Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, et al. (March 2005). "The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility". Science. 307 (5714): 1434–1440. Bibcode: 2005Sci...307.1434G. doi: 10.1126/science.1101160. PMID 15637236. S2CID 8815153.
- ^ a b Liu S, Yao L, Ding D, Zhu H (December 2010). "CCL3L1 copy number variation and susceptibility to HIV-1 infection: a meta-analysis". PLOS ONE. 5 (12): e15778. Bibcode: 2010PLoSO...515778L. doi: 10.1371/journal.pone.0015778. PMC 3012711. PMID 21209899.
- ^ Dube S, Qin J, Ramakrishnan R (August 2008). "Mathematical analysis of copy number variation in a DNA sample using digital PCR on a nanofluidic device". PLOS ONE. 3 (8): e2876. Bibcode: 2008PLoSO...3.2876D. doi: 10.1371/journal.pone.0002876. PMC 2483940. PMID 18682853.
- ^ Hughesman CB, Lu XJ, Liu KY, Zhu Y, Towle RM, Haynes C, Poh CF (September 2017). "Detection of clinically relevant copy number alterations in oral cancer progression using multiplexed droplet digital PCR". Scientific Reports. 7 (1): 11855. Bibcode: 2017NatSR...711855H. doi: 10.1038/s41598-017-11201-4. PMC 5605662. PMID 28928368.
- ^ a b Usher CL, Handsaker RE, Esko T, Tuke MA, Weedon MN, Hastie AR, et al. (August 2015). "Structural forms of the human amylase locus and their relationships to SNPs, haplotypes and obesity". Nature Genetics. 47 (8): 921–925. doi: 10.1038/ng.3340. PMC 4712930. PMID 26098870.
- ^ Aldhous MC, Abu Bakar S, Prescott NJ, Palla R, Soo K, Mansfield JC, et al. (December 2010). "Measurement methods and accuracy in copy number variation: failure to replicate associations of beta-defensin copy number with Crohn's disease". Human Molecular Genetics. 19 (24): 4930–4938. doi: 10.1093/hmg/ddq411. PMC 2989891. PMID 20858604.
- ^ Pinheiro L, Emslie KR (2018). "Basic Concepts and Validation of Digital PCR Measurements". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 11–24. doi: 10.1007/978-1-4939-7778-9_2. ISBN 978-1-4939-7776-5. PMID 29717435.
- ^ Handsaker RE, Van Doren V, Berman JR, Genovese G, Kashin S, Boettger LM, McCarroll SA (March 2015). "Large multiallelic copy number variations in humans". Nature Genetics. 47 (3): 296–303. doi: 10.1038/ng.3200. PMC 4405206. PMID 25621458.
- ^ Garcia-Murillas I, Turner NC (2018). "Assessing HER2 Amplification in Plasma cfDNA". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 161–172. doi: 10.1007/978-1-4939-7778-9_10. ISBN 978-1-4939-7776-5. PMID 29717443.
- ^ Christgen M, van Luttikhuizen JL, Raap M, Braubach P, Schmidt L, Jonigk D, et al. (December 2016). "Precise ERBB2 copy number assessment in breast cancer by means of molecular inversion probe array analysis". Oncotarget. 7 (50): 82733–82740. doi: 10.18632/oncotarget.12421. PMC 5347728. PMID 27716627.
- ^ Borley A, Mercer T, Morgan M, Dutton P, Barrett-Lee P, Brunelli M, Jasani B (April 2014). "Impact of HER2 copy number in IHC2+/FISH-amplified breast cancer on outcome of adjuvant trastuzumab treatment in a large UK cancer network". British Journal of Cancer. 110 (8): 2139–2143. doi: 10.1038/bjc.2014.147. PMC 3992505. PMID 24691421.
- ^ Cao Y, Raith MR, Griffith JF (March 2015). "Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment". Water Research. 70: 337–349. Bibcode: 2015WatRe..70..337C. doi: 10.1016/j.watres.2014.12.008. PMID 25543243.
- ^ European Society for Medical Oncology (17 Nov 2017). "Study analyzes mutations in cerebrospinal fluid in lung cancer with brain metastases". Retrieved 24 July 2019.
- ^ a b Petrone J (8 June 2017). "Norwegian Team Plans to Debut Digital PCR-Based Urinary Bladder Cancer Test by Year End". Retrieved 24 July 2019.
- ^ Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, Jiwa NM, de Boer M, Leguit RJ, et al. (April 2018). "The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid". Hematological Oncology. 36 (2): 429–435. doi: 10.1002/hon.2489. PMID 29210102. S2CID 4968214.
- ^ a b Paxton A (October 2017). "Revived hopes, fresh challenges with liquid biopsy". Retrieved 24 July 2019.
- ^ Bhadra K, Mellert H, Pestano G (5 Jun 2017). "Adoption of Liquid Biopsy Tests for NSCLC". Retrieved 24 July 2019.
- ^ Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, et al. (August 2016). "Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer". JAMA Oncology. 2 (8): 1014–1022. doi: 10.1001/jamaoncol.2016.0173. PMC 4982795. PMID 27055085.
- ^ Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, et al. (August 2015). "Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease". EMBO Molecular Medicine. 7 (8): 1034–1047. doi: 10.15252/emmm.201404913. PMC 4551342. PMID 25987569.
- ^ Carpinetti P, Donnard E, Bettoni F, Asprino P, Koyama F, Rozanski A, et al. (November 2015). "The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation". Oncotarget. 6 (35): 38360–38371. doi: 10.18632/oncotarget.5256. PMC 4742005. PMID 26451609.
- ^ Reinert T, Schøler LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, et al. (April 2016). "Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery". Gut. 65 (4): 625–634. doi: 10.1136/gutjnl-2014-308859. PMID 25654990.
- ^ Pereira E, Camacho-Vanegas O, Anand S, Sebra R, Catalina Camacho S, Garnar-Wortzel L, et al. (2015). "Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers". PLOS ONE. 10 (12): e0145754. Bibcode: 2015PLoSO..1045754P. doi: 10.1371/journal.pone.0145754. PMC 4696808. PMID 26717006.
- ^ Dahmcke CM, Steven KE, Larsen LK, Poulsen AL, Abdul-Al A, Dahl C, Guldberg P (December 2016). "A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria". European Urology. 70 (6): 916–919. doi: 10.1016/j.eururo.2016.06.035. PMID 27417036.
- ^ a b c Lindner L, Cayrou P, Jacquot S, Birling MC, Herault Y, Pavlovic G (July 2021). "Reliable and robust droplet digital PCR (ddPCR) and RT-ddPCR protocols for mouse studies". Methods. 191: 95–106. doi: 10.1016/j.ymeth.2020.07.004. PMID 32721466. S2CID 220851187.
- ^ Taylor SC, Carbonneau J, Shelton DN, Boivin G (November 2015). "Optimization of Droplet Digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: Clinical implications for quantification of Oseltamivir-resistant subpopulations". Journal of Virological Methods. 224: 58–66. doi: 10.1016/j.jviromet.2015.08.014. PMID 26315318.
- ^ Whale AS, Huggett JF, Tzonev S (December 2016). "Fundamentals of multiplexing with digital PCR". Biomolecular Detection and Quantification. 10: 15–23. doi: 10.1016/j.bdq.2016.05.002. PMC 5154634. PMID 27990345.
- ^ Sun B, Tao L, Zheng YL (June 2014). "Simultaneous quantification of alternatively spliced transcripts in a single droplet digital PCR reaction". BioTechniques. 56 (6): 319–325. doi: 10.2144/000114179. PMID 24924392.
- ^ Valencia CA, Rhodenizer D, Bhide S, Chin E, Littlejohn MR, Keong LM, et al. (2012). "Assessment of target enrichment platforms using massively parallel sequencing for the mutation detection for congenital muscular dystrophy". The Journal of Molecular Diagnostics. 14 (3): 233–246. doi: 10.1016/j.jmoldx.2012.01.009. PMC 3349841. PMID 22426012.
- ^ Philippe J, Derhourhi M, Durand E, Vaillant E, Dechaume A, Rabearivelo I, et al. (2015). "What Is the Best NGS Enrichment Method for the Molecular Diagnosis of Monogenic Diabetes and Obesity?". PLOS ONE. 10 (11): e0143373. Bibcode: 2015PLoSO..1043373P. doi: 10.1371/journal.pone.0143373. PMC 4657897. PMID 26599467.
- ^ Ouellet E, Foley JH, Conway EM, Haynes C (August 2015). "Hi-Fi SELEX: A High-Fidelity Digital-PCR Based Therapeutic Aptamer Discovery Platform". Biotechnology and Bioengineering. 112 (8): 1506–1522. doi: 10.1002/bit.25581. PMID 25727321. S2CID 39450798.
- ^ Ludlow AT, Shelton D, Wright WE, Shay JW (2018). "DdTRAP: A Method for Sensitive and Precise Quantification of Telomerase Activity". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 513–529. doi: 10.1007/978-1-4939-7778-9_29. ISBN 978-1-4939-7776-5. PMC 6046637. PMID 29717462.
- ^ Sayed ME, Slusher AL, Ludlow AT (May 2019). "Droplet Digital TRAP (ddTRAP): Adaptation of the Telomere Repeat Amplification Protocol to Droplet Digital Polymerase Chain Reaction". Journal of Visualized Experiments (147). doi: 10.3791/59550. PMID 31107456. S2CID 155519448.
- ^ Stein RA (1 July 2019). "Single-Cell Sequencing Sifts through Multiple Omics". Retrieved 1 August 2019.
- ^ Wood-Bouwens CM, Ji HP (2018). "Single Color Multiplexed DDPCR Copy Number Measurements and Single Nucleotide Variant Genotyping". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 323–333. doi: 10.1007/978-1-4939-7778-9_18. ISBN 978-1-4939-7776-5. PMID 29717451.
- ^ Low, H., Chan, SJ., Soo, GH. et al. Clarity™ digital PCR system: a novel platform for absolute quantification of nucleic acids. Anal Bioanal Chem 409, 1869–1875 (2017). https://doi.org/10.1007/s00216-016-0131-7
- ^ Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. (January 1988). "Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase". Science. 239 (4839): 487–491. Bibcode: 1988Sci...239..487S. doi: 10.1126/science.239.4839.487. PMID 2448875.
- ^ Morley AA (September 2014). "Digital PCR: A brief history". Biomolecular Detection and Quantification. 1 (1): 1–2. doi: 10.1016/j.bdq.2014.06.001. PMC 5129430. PMID 27920991.
- ^ Rutsaert S, Bosman K, Trypsteen W, Nijhuis M, Vandekerckhove L (January 2018). "Digital PCR as a tool to measure HIV persistence". Retrovirology. 15 (1): 16. doi: 10.1186/s12977-018-0399-0. PMC 5789538. PMID 29378600.
- ^ a b Perkel J (11 April 2014). "The digital PCR revolution". Retrieved 22 July 2019.
- ^ Pohl G, Shih I (January 2004). "Principle and applications of digital PCR". Expert Review of Molecular Diagnostics. 4 (1): 41–47. doi: 10.1586/14737159.4.1.41. PMID 14711348. S2CID 28271641.
- ^ Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B (July 2003). "Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations". Proceedings of the National Academy of Sciences of the United States of America. 100 (15): 8817–8822. Bibcode: 2003PNAS..100.8817D. doi: 10.1073/pnas.1133470100. PMC 166396. PMID 12857956.
- ^ Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D (July 2006). "BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions". Nature Methods. 3 (7): 551–559. doi: 10.1038/nmeth898. PMID 16791214. S2CID 7059151.
- ^ Butkus B (8 July 2010). "Digital PCR Space Heating Up as Life Science Tool Vendors Begin Staking Claims". Retrieved 22 July 2019.
- ^ Ramakrishnan R, Qin J, Jones RC, Weaver LS (2013). "Integrated Fluidic Circuits (IFCs) for Digital PCR". Microfluidic Diagnostics. Methods in Molecular Biology. Vol. 949. pp. 423–431. doi: 10.1007/978-1-62703-134-9_27. ISBN 978-1-62703-133-2. PMID 23329458.
- ^ Butkus B (29 Mar 2012). "RainDance Launches Digital PCR Platform; Claims Sensitivity, Operating Cost Superiority". Retrieved 22 July 2019.
- ^ "'Liquid biopsy' blood test detects genetic mutations in common form of lung cancer". 7 Apr 2016. Retrieved 22 July 2019.
- ^ "Korea's BioCore First to Commercialize NIPT Based on Digital PCR". 2 Mar 2018. Retrieved 22 July 2019.
- ^ "Bio-Rad Gets First CE Mark on Clinical ddPCR Test". 5 Dec 2017. Retrieved 22 Jul 2019.
A major contributor to this article appears to have a
close connection with its subject. (August 2019) |
Digital polymerase chain reaction (digital PCR, DigitalPCR, dPCR, or dePCR) is a biotechnological refinement of conventional polymerase chain reaction methods that can be used to directly quantify and clonally amplify nucleic acids strands including DNA, cDNA, or RNA. The key difference between dPCR and qPCR lies in the method of measuring nucleic acids amounts, with the former being a more precise method than PCR, though also more prone to error in the hands of inexperienced users. [1] PCR carries out one reaction per single sample. dPCR also carries out a single reaction within a sample, however the sample is separated into a large number of partitions and the reaction is carried out in each partition individually. This separation allows a more reliable collection and sensitive measurement of nucleic acid amounts. The method has been demonstrated as useful for studying variations in gene sequences — such as copy number variants and point mutations.

The polymerase chain reaction method is used to quantify nucleic acids by amplifying a nucleic acid molecule with the enzyme DNA polymerase. [2] Conventional PCR is based on the theory that amplification is exponential. Therefore, nucleic acids may be quantified by comparing the number of amplification cycles and amount of PCR end-product to those of a reference sample. [3] However, many factors complicate this calculation, creating uncertainties and inaccuracies. These factors include the following: initial amplification cycles may not be exponential; PCR amplification eventually plateaus after an uncertain number of cycles; and low initial concentrations of target nucleic acid molecules may not amplify to detectable levels. However, the most significant limitation of PCR is that PCR amplification efficiency in a sample of interest may be different from that of reference samples.


Instead of performing one reaction per well, dPCR involves partitioning the PCR solution into tens of thousands of nano-liter sized droplets, where a separate PCR reaction takes place in each one. [4] [5] A PCR solution is made similarly to a TaqMan assay, which consists of template DNA (or RNA), fluorescence-quencher probes, primers, and a PCR master mix, which contains DNA polymerase, dNTPs, MgCl2, and reaction buffers at optimal concentrations. Several different methods can be used to partition samples, including microwell plates, capillaries, oil emulsion, and arrays of miniaturized chambers with nucleic acid binding surfaces. [6] The PCR solution is partitioned into smaller units, each with the necessary components for amplification. The partitioned units are then subjected to thermocycling so that each unit may independently undergo PCR amplification. After multiple PCR amplification cycles, the samples are checked for fluorescence with a binary readout of “0” or “1”. The fraction of fluorescing droplets is recorded. [5] The partitioning of the sample allows one to estimate the number of different molecules by assuming that the molecule population follows the Poisson distribution, thus accounting for the possibility of multiple target molecules inhabiting a single droplet. Using Poisson's law of small numbers, the distribution of target molecule within the sample can be accurately approximated allowing for a quantification of the target strand in the PCR product. [7] This model simply predicts that as the number of samples containing at least one target molecule increases, the probability of the samples containing more than one target molecule increases. [8] In conventional PCR, the number of PCR amplification cycles is proportional to the starting copy number. Different from many people's belief that dPCR provides absolute quantification, digital PCR uses statistical power to provide relative quantification. For example, if Sample A, when assayed in 1 million partitions, gives one positive reaction, it does not mean that the Sample A has one starting molecule.[ citation needed]
The benefits of dPCR include increased precision through massive sample partitioning, which ensures reliable measurements in the desired DNA sequence due to reproducibility. [5] Error rates are larger when detecting small-fold change differences with basic PCR, while error rates are smaller with dPCR due to the smaller-fold change differences that can be detected in DNA sequence. The technique itself reduces the use of a larger volume of reagent needed, which inevitably will lower experiment cost. Also, dPCR is highly quantitative as it does not rely on relative fluorescence of the solution to determine the amount of amplified target DNA.
dPCR measures the actual number of molecules (target DNA) as each molecule is in one droplet, thus making it a discrete “digital” measurement. It provides absolute quantification because dPCR measures the positive fraction of samples, which is the number of droplets that are fluorescing due to proper amplification. This positive fraction accurately indicates the initial amount of template nucleic acid. Similarly, qPCR utilizes fluorescence; however, it measures the intensity of fluorescence at specific times (generally after every amplification cycle) to determine the relative amount of target molecule (DNA), but cannot specify the exact amount without constructing a standard curve using different amounts of a defined standard. It gives the threshold per cycle (CT) and the difference in CT is used to calculate the amount of initial nucleic acid. As such, qPCR is an analog measurement, which may not be as precise due to the extrapolation required to attain a measurement. [6] [9]
dPCR measures the amount of DNA after amplification is complete and then determines the fraction of replicates. This is representative of an endpoint measurement as it requires the observation of the data after the experiment is completed. In contrast, qPCR records the relative fluorescence of the DNA at specific points during the amplification process, which requires stops in the experimental process. This “real-time” aspect of qPCR may theoretically affect results due to the stopping of the experiment.[ citation needed] In practice, however, most qPCR thermal cyclers read each sample's fluorescence very quickly at the end of the annealing/extension step before proceeding to the next melting step, meaning this hypothetical concern is not actually relevant or applicable for the vast majority of researchers. dPCR measures the amplification by measuring the products of end point PCR cycling and is therefore less susceptible to the artifacts arising from impaired amplification efficiencies due to the presence of PCR inhibitors or primer template mismatch. [10] [11]
Real-time Digital PCR (rdPCR) combines the methodologies of digital PCR (dPCR) and quantitative PCR (qPCR), integrating the precision of dPCR with the real-time analysis capabilities of qPCR. This integration aims to provide enhanced sensitivity, specificity, and the ability for absolute quantification of nucleic acid sequences, contributing to the quantification of genetic material in scientific and clinical research. [12] [13]
qPCR is unable to distinguish differences in gene expression or copy number variations that are smaller than twofold. On the other hand, dPCR has a higher precision and has been shown to detect differences of less than 30% in gene expression, distinguish between copy number variations that differ by only 1 copy, and identify alleles that occur at frequencies less than 0.1%. [14] [5]
Digital PCR has many applications in basic research, clinical diagnostics and environmental testing. Its uses include pathogen detection and digestive health analysis; [15] [16] liquid biopsy for cancer monitoring, organ transplant rejection monitoring and non-invasive prenatal testing for serious genetic abnormalities; [17] [18] [19] [20] [21] [22] [23] [24] copy number variation analysis, [25] [26] [27] single gene expression analysis, [28] rare sequence detection, [24] [29] [30] gene expression profiling and single-cell analysis; [31] [32] [30] [33] [34] [35] [36] the detection of DNA contaminants in bioprocessing, [37] the validation of gene edits and detection of specific methylation changes in DNA as biomarkers of cancer. [38] [39] [40] dPCR is also frequently used as an orthogonal method to confirm rare mutations detected through next-generation sequencing (NGS) and to validate NGS libraries. [41] [42] [43]
dPCR enables the absolute and reproducible quantification of target nucleic acids at single-molecule resolution. [30] [44] [45] [46] Unlike analogue quantitative PCR (qPCR), however, absolute quantification with dPCR does not require a standard curve. [44] dPCR also has a greater tolerance for inhibitor substances and PCR assays that amplify inefficiently as compared to qPCR. [47] [11]
dPCR can quantify, for example, the presence of specific sequences from contaminating genetically modified organisms in foodstuffs, [48] viral load in the blood, [49] PBMCs, [50] [51] serum samples, [52] chorionic villi tissues, [50] [51] biomarkers of neurodegenerative disease in cerebral spinal fluid, [53] and fecal contamination in drinking water. [54]
An alteration in copy number state with respect to a single-copy reference locus is referred to as a “ copy number variation” (CNV) if it appears in germline cells, or a copy number alteration (CNA) if it appears in somatic cells. [55] A CNV or CNA could be due to a deletion or amplification of a locus with respect to the number of copies of the reference locus present in the cell, and together, they are major contributors to variability in the human genome. [56] [57] [58] They have been associated with cancers; [59] [60] [61] neurological, [62] psychiatric, [63] [64] and autoimmune diseases; [65] and adverse drug reactions. [66] However, it is difficult to measure these allelic variations with high precision using other methods such as qPCR, thus making phenotypic and disease associations with altered CNV status challenging. [67] [68]
The large number of “digitized,” endpoint measurements made possible by sample partitioning enables dPCR to resolve small differences in copy number with better accuracy and precision when compared to other methods such as SNP-based microarrays [69] or qPCR. [70] [71] qPCR is limited in its ability to precisely quantify gene amplifications in several diseases, including Crohn’s disease, HIV-1 infection, and obesity. [72] [68] [71]
dPCR was designed to measure the concentration of a nucleic acid target in copies per unit volume of the sample. When operating in dilute reactions where less than ~10% of the partitions contain a desired target (referred to as “limiting dilution”), copy number can be estimated by comparing the number of fluorescent droplets arising from a target CNV with the number of fluorescent droplets arising from an invariant single-copy reference locus. [25] In fact, both at these lower target concentrations and at higher ones where multiple copies of the same target can co-localize to a single partition, Poisson statistics are used to correct for these multiple occupancies to give a more accurate value for each target’s concentration. [73] [6]
Digital PCR has been used to uncover both germline and somatic variation in gene copy number between humans [74] and to study the link between amplification of HER2 (ERBB2) and breast cancer progression. [75] [76] [77] [27]
Partitioning in digital PCR increases sensitivity and allows for detection of rare events, especially single nucleotide variants (SNVs), by isolating or greatly diminishing the target biomarker signal from potentially competing background. [9] [6] These events can be organized into two classes: rare mutation detection and rare sequence detection.
Rare mutation detection occurs when a biomarker exists within a background of a highly abundant counterpart that differs by only a single nucleotide variant (SNV). Digital PCR has been shown to be capable of detecting mutant DNA in the presence of a 200,000-fold excess of wild type background, which is 2,000 times more sensitive than achievable with conventional qPCR. [9]
Digital PCR can detect rare sequences such as HIV DNA in patients with HIV, [24] and DNA from fecal bacteria in ocean and other water samples for assessing water quality. [78] dPCR can detect sequences as rare as 1 in every 1,250,000 cells. [24]
dPCR’s ability to detect rare mutations may be of particular benefit in the clinic through the use of the liquid biopsy, a generally noninvasive strategy for detecting and monitoring disease via bodily fluids. [17] [79] Researchers have used liquid biopsy to monitor tumor load, treatment response and disease progression in cancer patients by measuring rare mutations in circulating tumor DNA (ctDNA) in a variety of biological fluids from patients including blood, urine and cerebrospinal fluid. [17] [80] [81] Early detection of ctDNA (as in molecular relapse) may lead to earlier administration of an immunotherapy or a targeted therapy specific for the patient’s mutation signature, potentially improving chances of the treatment’s effectiveness rather than waiting for clinical relapse before altering treatment. Liquid biopsies can have turnaround times of a few days, compared to two to four weeks or longer for tissue-based tests. [82] [83] This reduced time to results has been used by physicians to expedite treatments tailored to biopsy data. [82]
In 2016, a prospective trial using dPCR at the Dana-Farber Cancer Institute authenticated the clinical benefit of liquid biopsy as a predictive diagnostic tool for patients with non-small-cell lung cancer. [84] The application of liquid biopsy tests have also been studied in patients with breast, [85] colorectal, [86] [87] gynecologic, [88] and bladder cancers [80] [89] to monitor both the disease load and the tumor’s response to treatment.
Gene expression and RNA quantification studies have benefited from the increased precision and absolute quantification of dPCR. [90] RNA quantification can be accomplished via RT-PCR, wherein RNA is reverse-transcribed into cDNA in the partitioned reaction itself, and the number of RNA molecules originating from each transcript (or allelic transcript) is quantified via dPCR. [31]
One can often achieve greater sensitivity and precision by using dPCR rather than qPCR to quantify RNA molecules in part because it does not require use of a standard curve for quantification. [91] dPCR is also more resilient to PCR inhibitors for the quantification of RNA than qPCR. [47] [16] [90]
dPCR can detect and quantify more individual target species per detection channel than qPCR by virtue of being able to distinguish targets based on their differential fluorescence amplitude or by the use of distinctive color combinations for their detection. [92] [90] As an example of this, a 2-channel dPCR system has been used to detect in a single well the expression of four different splice variants of human telomerase reverse transcriptase, a protein that is more active in most tumor cells than in healthy cells. [93]
Using the dynamic partitioning capabilities employed in dPCR, improved NGS sequencing can be achieved by partitioning of complex PCR reactions prior to amplification to give more uniform amplification across many distinct amplicons for NGS analysis. [94] [95] Additionally, the improved specificity of complex PCR amplification reactions in droplets has been shown to greatly reduce the number of iterations required to select for high affinity aptamers in the SELEX method. [96] Partitioning can also allow for more robust measurements of telomerase activity from cell lysates. [97] [98] dPCR’s dynamic partitioning capabilities can also be used to partition thousands of nuclei or whole cells into individual droplets to facilitate library preparation for a single cell assay for transposase-accessible chromatin using sequencing (scATAC-seq). [99]
Droplet Digital PCR (ddPCR) is a method of dPCR in which a 20 microliter sample reaction including assay primers and either Taqman probes or an intercalating dye, is divided into ~20,000 nanoliter-sized oil droplets through a water-oil emulsion technique, thermocycled to endpoint in a 96-well PCR plate, and fluorescence amplitude read for all droplets in each sample well in a droplet flow cytometer. [100]
Chip-based Digital PCR (dPCR) is also a method of dPCR in which the reaction mix (also when used in qPCR) is divided into ~10,000 to ~45,000 partitions on a chip, then amplified using an endpoint PCR thermocycling machine, and is read using a high-powered camera reader with fluorescence filter (HEX, FAM, Cy5, Cy5.5 and Texas Red) for all partitions on each chip. [101]
dPCR rose out of an approach first published in 1988 by Cetus Corporation when researchers showed that a single copy of the β-globin gene could be detected and amplified by PCR. [102] [103] This was achieved by diluting DNA samples from a normal human cell line with DNA from a mutant line having a homozygous deletion of the β-globin gene, until it was no longer present in the reaction. In 1989, Peter Simmonds, AJ Brown et al. used this concept to quantify a molecule for the first time. [104] Alex Morley and Pamela Sykes formally established the method as a quantitative technique in 1992. [45]
In 1999, Bert Vogelstein and Kenneth Kinzler coined the term “digital PCR” and showed that the technique could be used to find rare cancer mutations. [105] However, dPCR was difficult to perform; it was labor intensive, required a lot of training to do properly, and was difficult to do in large quantities. [105] In 2003, Kinzler and Vogelstein continued to refine dPCR and created an improved method that they called BEAMing technology, an acronym for “beads, emulsion, amplification and magnetics.” The new protocol used emulsion to compartmentalize amplification reactions in a single tube. This change made it possible for scientists to scale the method to thousands of reactions in a single run. [106] [107] [108]
Companies developing commercial dPCR systems have integrated technologies like automated partitioning of samples, digital counting of nucleic acid targets, and increasing droplet count that can help the process be more efficient. [109] [110] [111] In recent years, scientists have developed and commercialized dPCR-based diagnostics for several conditions, including non-small cell lung cancer and Down’s Syndrome. [112] [113] The first dPCR system for clinical use was CE-marked in 2017 and cleared by the US Food and Drug Administration in 2019, for diagnosing chronic myeloid leukemia. [114]
- ^ Perkel J (May 2015). "Guiding our PCR experiments". BioTechniques. 58 (5): 217–221. doi: 10.2144/000114283. PMID 25967899.
- ^ "Polymerase Chain Reaction (PCR)". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Higuchi, Russell; Fockler, Carita; Dollinger, Gavin; Watson, Robert (September 1993). "Kinetic PCR Analysis: Real-time Monitoring of DNA Amplification Reactions". Bio/Technology. 11 (9): 1026–1030. doi: 10.1038/nbt0993-1026. ISSN 1546-1696. PMID 7764001. S2CID 5714001.
- ^ Duewer DL, Kline MC, Romsos EL, Toman B (May 2018). "Evaluating droplet digital PCR for the quantification of human genomic DNA: converting copies per nanoliter to nanograms nuclear DNA per microliter". Analytical and Bioanalytical Chemistry. 410 (12): 2879–2887. doi: 10.1007/s00216-018-0982-1. PMC 5996397. PMID 29556737.
- ^ a b c d Baker M (2012). "Digital PCR hits its stride". Nature Methods. 9 (6): 541–544. doi: 10.1038/nmeth.2027. S2CID 46347563.
- ^ a b c d Quan PL, Sauzade M, Brouzes E (April 2018). "dPCR: A Technology Review". Sensors. 18 (4): 1271. Bibcode: 2018Senso..18.1271Q. doi: 10.3390/s18041271. PMC 5948698. PMID 29677144.
- ^ Prediger E. "Digital PCR (dPCR)—What is it and why use it?". Integrated DNA Technologies.
- ^ Butler DM, Pacold ME, Jordan PS, Richman DD, Smith DM (December 2009). "The efficiency of single genome amplification and sequencing is improved by quantitation and use of a bioinformatics tool". Journal of Virological Methods. 162 (1–2): 280–283. doi: 10.1016/j.jviromet.2009.08.002. PMC 2761514. PMID 19698751.
- ^ a b c Pekin D, Skhiri Y, Baret JC, Le Corre D, Mazutis L, Salem CB, et al. (July 2011). "Quantitative and sensitive detection of rare mutations using droplet-based microfluidics". Lab on a Chip. 11 (13): 2156–2166. doi: 10.1039/c1lc20128j. PMID 21594292.
- ^ Svec D, Tichopad A, Novosadova V, Pfaffl MW, Kubista M (March 2015). "How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments". Biomolecular Detection and Quantification. 3: 9–16. doi: 10.1016/j.bdq.2015.01.005. PMC 4822216. PMID 27077029.
- ^ a b Dingle TC, Sedlak RH, Cook L, Jerome KR (November 2013). "Tolerance of droplet-digital PCR vs real-time quantitative PCR to inhibitory substances". Clinical Chemistry. 59 (11): 1670–1672. doi: 10.1373/clinchem.2013.211045. PMC 4247175. PMID 24003063.
- ^ Pavšič, Jernej; Žel, Jana; Milavec, Mojca (2016-01-01). "Assessment of the real-time PCR and different digital PCR platforms for DNA quantification". Analytical and Bioanalytical Chemistry. 408 (1): 107–121. doi: 10.1007/s00216-015-9107-2. ISSN 1618-2650. PMC 4706846. PMID 26521179.
- ^ Xu, Jiachen; Duong, Kyra; Yang, Zhenlin; Kaji, Kavanaugh; Ou, Jiajia; Head, Steven R.; Crynen, Gogce; Ordoukhanian, Phillip; Hanna, Lauren; Hanna, Ava; Wang, Yan; Wang, Zhijie; Wang, Jie (December 2021). "Real-time digital polymerase chain reaction (PCR) as a novel technology improves limit of detection for rare allele assays". Translational Lung Cancer Research. 10 (12): 4336–4352. doi: 10.21037/tlcr-21-728. ISSN 2226-4477. PMC 8743530. PMID 35070745.
- ^ Salipante SJ, Jerome KR (January 2020). "Digital PCR-An Emerging Technology with Broad Applications in Microbiology". Clinical Chemistry. 66 (1): 117–123. doi: 10.1373/clinchem.2019.304048. PMID 31704712.
- ^ Witte AK, Fister S, Mester P, Schoder D, Rossmanith P (November 2016). "Evaluation of the performance of quantitative detection of the Listeria monocytogenes prfA locus with droplet digital PCR". Analytical and Bioanalytical Chemistry. 408 (27): 7583–7593. doi: 10.1007/s00216-016-9861-9. PMC 5061835. PMID 27558101.
- ^ a b Stauber J, Shaikh N, Ordiz MI, Tarr PI, Manary MJ (May 2016). "Droplet digital PCR quantifies host inflammatory transcripts in feces reliably and reproducibly". Cellular Immunology. 303: 43–49. doi: 10.1016/j.cellimm.2016.03.007. PMC 4863679. PMID 27063479.
- ^ a b c Skibo S (23 Feb 2018). "Has Tumor Profiling Caught Up to Cancer?". Retrieved 23 July 2019.
- ^ Hirsch F (27 July 2018). "Guidelines highlight 'best practices' for liquid biopsy during treatment of non-small cell lung cancer". Retrieved 23 July 2019.
- ^ Johnson M (12 Jan 2018). "Bio-Rad Continues to Advance Digital PCR Tech, Liquid Biopsy Tests Into Commercial Clinical Market". Retrieved 23 July 2019.
- ^ Oxnard GR, Paweletz CP, Kuang Y, Mach SL, O'Connell A, Messineo MM, et al. (March 2014). "Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA". Clinical Cancer Research. 20 (6): 1698–1705. doi: 10.1158/1078-0432.CCR-13-2482. PMC 3959249. PMID 24429876.
- ^ Schütz E, Fischer A, Beck J, Harden M, Koch M, Wuensch T, et al. (April 2017). "Graft-derived cell-free DNA, a noninvasive early rejection and graft damage marker in liver transplantation: A prospective, observational, multicenter cohort study". PLOS Medicine. 14 (4): e1002286. doi: 10.1371/journal.pmed.1002286. PMC 5404754. PMID 28441386.
- ^ Lee SY, Hwang SY (2015). "Application of digital polymerase chain reaction technology for noninvasive prenatal test". Journal of Genetic Medicine. 12 (2): 72–78. doi: 10.5734/JGM.2015.12.2.72. ISSN 2383-8442.
- ^ Gu W, Koh W, Blumenfeld YJ, El-Sayed YY, Hudgins L, Hintz SR, Quake SR (July 2014). "Noninvasive prenatal diagnosis in a fetus at risk for methylmalonic acidemia". Genetics in Medicine. 16 (7): 564–567. doi: 10.1038/gim.2013.194. PMC 4079742. PMID 24406457.
- ^ a b c d Strain MC, Lada SM, Luong T, Rought SE, Gianella S, Terry VH, et al. (2013). "Highly precise measurement of HIV DNA by droplet digital PCR". PLOS ONE. 8 (4): e55943. Bibcode: 2013PLoSO...855943S. doi: 10.1371/journal.pone.0055943. PMC 3616050. PMID 23573183.
- ^
a
b Bell AD, Usher CL, McCarroll SA (2018). "Analyzing Copy Number Variation with Droplet Digital PCR". Digital PCR. Methods in Molecular Biology. Vol. 1768. Clifton, N.J. pp. 143–160.
doi:
10.1007/978-1-4939-7778-9_9.
ISBN
978-1-4939-7776-5.
PMID
29717442.
{{ cite book}}: CS1 maint: location missing publisher ( link) - ^ Shoda K, Ichikawa D, Fujita Y, Masuda K, Hiramoto H, Hamada J, et al. (January 2017). "Monitoring the HER2 copy number status in circulating tumor DNA by droplet digital PCR in patients with gastric cancer". Gastric Cancer. 20 (1): 126–135. doi: 10.1007/s10120-016-0599-z. PMID 26874951.
- ^ a b Gevensleben H, Garcia-Murillas I, Graeser MK, Schiavon G, Osin P, Parton M, et al. (June 2013). "Noninvasive detection of HER2 amplification with plasma DNA digital PCR". Clinical Cancer Research. 19 (12): 3276–3284. doi: 10.1158/1078-0432.CCR-12-3768. PMC 6485473. PMID 23637122.
- ^ Mazzoni E, Frontini F, Rotondo JC, Zanotta N, Fioravanti A, Minelli F, et al. (April 2019). "Antibodies reacting to mimotopes of Simian virus 40 large T antigen, the viral oncoprotein, in sera from children". Journal of Cellular Physiology. 234 (4): 3170–3179. doi: 10.1002/jcp.27490. hdl: 11392/2397717. PMID 30362540. S2CID 53106591.
- ^ Uchiyama Y, Nakashima M, Watanabe S, Miyajima M, Taguri M, Miyatake S, et al. (March 2016). "Ultra-sensitive droplet digital PCR for detecting a low-prevalence somatic GNAQ mutation in Sturge-Weber syndrome". Scientific Reports. 6 (1): 22985. Bibcode: 2016NatSR...622985U. doi: 10.1038/srep22985. PMC 4783707. PMID 26957145.
- ^ a b c Marusina K (1 Oct 2017). "Positioning Digital PCR for Sharper Genomic Views". Retrieved 23 July 2019.
- ^ a b Kamitaki N, Usher CL, McCarroll SA (2018). "Using Droplet Digital PCR to Analyze Allele-Specific RNA Expression". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 401–422. doi: 10.1007/978-1-4939-7778-9_23. ISBN 978-1-4939-7776-5. PMID 29717456.
- ^ Millier MJ, Stamp LK, Hessian PA (December 2017). "Digital-PCR for gene expression: impact from inherent tissue RNA degradation". Scientific Reports. 7 (1): 17235. Bibcode: 2017NatSR...717235M. doi: 10.1038/s41598-017-17619-0. PMC 5722939. PMID 29222437.
- ^ "Highly Sensitive Detection of Hepatitis B Using ddPCR". 12 Apr 2018. Retrieved 23 July 2019.
- ^ Jang M, Jeong SW, Bae NH, Song Y, Lee TJ, Lee MK, Lee SJ, Lee KG (2017). "Droplet-based digital PCR system for detection of single-cell level of foodborne pathogens". BioChip Journal. 11 (4): 329–337. doi: 10.1007/s13206-017-1410-x. ISSN 2092-7843. S2CID 89829687.
- ^ Igarashi Y, Uchiyama T, Minegishi T, Takahashi S, Watanabe N, Kawai T, et al. (September 2017). "Single Cell-Based Vector Tracing in Patients with ADA-SCID Treated with Stem Cell Gene Therapy". Molecular Therapy. Methods & Clinical Development. 6: 8–16. doi: 10.1016/j.omtm.2017.05.005. PMC 5466583. PMID 28626778.
- ^ Albayrak C, Jordi CA, Zechner C, Lin J, Bichsel CA, Khammash M, Tay S (March 2016). "Digital Quantification of Proteins and mRNA in Single Mammalian Cells". Molecular Cell. 61 (6): 914–924. doi: 10.1016/j.molcel.2016.02.030. PMID 26990994.
- ^ Hussain M, Fantuzzo R, Mercorelli S, Cullen C (May 2016). "A direct droplet digital PCR method for quantification of residual DNA in protein drugs produced in yeast cells". Journal of Pharmaceutical and Biomedical Analysis. 123: 128–131. doi: 10.1016/j.jpba.2016.01.050. PMID 26896631.
- ^ Miyaoka Y, Chan AH, Judge LM, Yoo J, Huang M, Nguyen TD, et al. (March 2014). "Isolation of single-base genome-edited human iPS cells without antibiotic selection". Nature Methods. 11 (3): 291–293. doi: 10.1038/nmeth.2840. PMC 4063274. PMID 24509632.
- ^ Nelson CE, Hakim CH, Ousterout DG, Thakore PI, Moreb EA, Castellanos Rivera RM, et al. (January 2016). "In vivo genome editing improves muscle function in a mouse model of Duchenne muscular dystrophy". Science. 351 (6271): 403–407. Bibcode: 2016Sci...351..403N. doi: 10.1126/science.aad5143. PMC 4883596. PMID 26721684.
- ^ Miyaoka Y, Berman JR, Cooper SB, Mayerl SJ, Chan AH, Zhang B, et al. (March 2016). "Systematic quantification of HDR and NHEJ reveals effects of locus, nuclease, and cell type on genome-editing". Scientific Reports. 6: 23549. Bibcode: 2016NatSR...623549M. doi: 10.1038/srep23549. PMC 4814844. PMID 27030102.
- ^ Guttery DS, Page K, Hills A, Woodley L, Marchese SD, Rghebi B, et al. (July 2015). "Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen receptor-positive metastatic breast cancer". Clinical Chemistry. 61 (7): 974–982. doi: 10.1373/clinchem.2015.238717. PMID 25979954.
- ^ Robin JD, Ludlow AT, LaRanger R, Wright WE, Shay JW (April 2016). "Comparison of DNA Quantification Methods for Next Generation Sequencing". Scientific Reports. 6 (1): 24067. Bibcode: 2016NatSR...624067R. doi: 10.1038/srep24067. PMC 4822169. PMID 27048884.
- ^ Aigrain L, Gu Y, Quail MA (June 2016). "Quantitation of next generation sequencing library preparation protocol efficiencies using droplet digital PCR assays - a systematic comparison of DNA library preparation kits for Illumina sequencing". BMC Genomics. 17 (1): 458. doi: 10.1186/s12864-016-2757-4. PMC 4906846. PMID 27297323.
- ^ a b Brunetto GS, Massoud R, Leibovitch EC, Caruso B, Johnson K, Ohayon J, et al. (August 2014). "Digital droplet PCR (ddPCR) for the precise quantification of human T-lymphotropic virus 1 proviral loads in peripheral blood and cerebrospinal fluid of HAM/TSP patients and identification of viral mutations". Journal of Neurovirology. 20 (4): 341–351. doi: 10.1007/s13365-014-0249-3. PMC 4085507. PMID 24781526.
- ^ a b Sykes PJ, Neoh SH, Brisco MJ, Hughes E, Condon J, Morley AA (September 1992). "Quantitation of targets for PCR by use of limiting dilution". BioTechniques. 13 (3): 444–449. PMID 1389177.
- ^ Vogelstein B, Kinzler KW (August 1999). "Digital PCR". Proceedings of the National Academy of Sciences of the United States of America. 96 (16): 9236–9241. Bibcode: 1999PNAS...96.9236V. doi: 10.1073/pnas.96.16.9236. PMC 17763. PMID 10430926.
- ^ a b Rački N, Dreo T, Gutierrez-Aguirre I, Blejec A, Ravnikar M (2014). "Reverse transcriptase droplet digital PCR shows high resilience to PCR inhibitors from plant, soil and water samples". Plant Methods. 10 (1): 42. doi: 10.1186/s13007-014-0042-6. PMC 4307183. PMID 25628753.
- ^ Dobnik D, Spilsberg B, Bogožalec Košir A, Štebih D, Morisset D, Holst-Jensen A, Žel J (2018). "Multiplex Droplet Digital PCR Protocols for Quantification of GM Maize Events". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 69–98. doi: 10.1007/978-1-4939-7778-9_5. ISBN 978-1-4939-7776-5. PMID 29717438.
- ^ Vellucci A, Leibovitch EC, Jacobson S (2018). "Using Droplet Digital PCR to Detect Coinfection of Human Herpesviruses 6A and 6B (HHV-6A and HHV-6B) in Clinical Samples". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 99–109. doi: 10.1007/978-1-4939-7778-9_6. ISBN 978-1-4939-7776-5. PMID 29717439.
- ^ a b Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Gonzalez LO, et al. (March 2020). "Droplet-digital PCR assay to detect Merkel cell polyomavirus sequences in chorionic villi from spontaneous abortion affected females". Journal of Cellular Physiology. 235 (3): 1888–1894. doi: 10.1002/jcp.29213. hdl: 11392/2409453. PMID 31549405.
- ^ a b Tagliapietra A, Rotondo JC, Bononi I, Mazzoni E, Magagnoli F, Maritati M, et al. (March 2019). "Footprints of BK and JC polyomaviruses in specimens from females affected by spontaneous abortion". Human Reproduction. 34 (3): 433–440. doi: 10.1002/jcp.27490. hdl: 11392/2397717. PMID 30590693. S2CID 53106591.
- ^ Mazzoni E, Rotondo JC, Marracino L, Selvatici R, Bononi I, Torreggiani E, et al. (2017). "Detection of Merkel Cell Polyomavirus DNA in Serum Samples of Healthy Blood Donors". Frontiers in Oncology. 7: 294. doi: 10.3389/fonc.2017.00294. PMC 5712532. PMID 29238698.
- ^ Podlesniy P, Trullas R (2018). "Biomarkers in Cerebrospinal Fluid: Analysis of Cell-Free Circulating Mitochondrial DNA by Digital PCR". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 111–126. doi: 10.1007/978-1-4939-7778-9_7. ISBN 978-1-4939-7776-5. PMID 29717440.
- ^ Cao Y, Raith MR, Griffith JF (2018). "Testing of General and Human-Associated Fecal Contamination in Waters". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 127–140. doi: 10.1007/978-1-4939-7778-9_8. ISBN 978-1-4939-7776-5. PMID 29717441.
- ^ Li W, Lee A, Gregersen PK (January 2009). "Copy-number-variation and copy-number-alteration region detection by cumulative plots". BMC Bioinformatics. 10 (S1): S67. arXiv: 0909.3129. Bibcode: 2009arXiv0909.3129L. doi: 10.1186/1471-2105-10-S1-S67. PMC 2648736. PMID 19208171.
- ^ Koren A, Handsaker RE, Kamitaki N, Karlić R, Ghosh S, Polak P, et al. (November 2014). "Genetic variation in human DNA replication timing". Cell. 159 (5): 1015–1026. doi: 10.1016/j.cell.2014.10.025. PMC 4359889. PMID 25416942.
- ^ Sanders S (16 Jul 2008). "CNVs vs SNPs: Understanding Human Structural Variation in Disease". Retrieved 24 July 2019.
- ^ Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. (January 2017). "Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects". Nature Genetics. 49 (1): 27–35. doi: 10.1038/ng.3725. PMC 5737772. PMID 27869829.
- ^ Shlien A, Malkin D (June 2009). "Copy number variations and cancer". Genome Medicine. 1 (6): 62. doi: 10.1186/gm62. PMC 2703871. PMID 19566914.
- ^ Lauer S, Gresham D (December 2019). "An evolving view of copy number variants". Current Genetics. 65 (6): 1287–1295. doi: 10.1007/s00294-019-00980-0. PMID 31076843. S2CID 149444714.
- ^ "Copy Number Alteration Found to Be Associated with Cancer Mortality". 5 Sep 2018. Retrieved 24 July 2019.
- ^ Gu W, Lupski JR (2008). "CNV and nervous system diseases--what's new?". Cytogenetic and Genome Research. 123 (1–4): 54–64. doi: 10.1159/000184692. PMC 2920183. PMID 19287139.
- ^ Thapar A, Cooper M (August 2013). "Copy number variation: what is it and what has it told us about child psychiatric disorders?". Journal of the American Academy of Child and Adolescent Psychiatry. 52 (8): 772–774. doi: 10.1016/j.jaac.2013.05.013. PMC 3919207. PMID 23880486.
- ^ Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, et al. (February 2016). "Schizophrenia risk from complex variation of complement component 4". Nature. 530 (7589): 177–183. Bibcode: 2016Natur.530..177.. doi: 10.1038/nature16549. PMC 4752392. PMID 26814963.
- ^ Yim SH, Jung SH, Chung B, Chung YJ (May 2015). "Clinical implications of copy number variations in autoimmune disorders". The Korean Journal of Internal Medicine. 30 (3): 294–304. doi: 10.3904/kjim.2015.30.3.294. PMC 4438283. PMID 25995659.
- ^ He Y, Hoskins JM, McLeod HL (May 2011). "Copy number variants in pharmacogenetic genes". Trends in Molecular Medicine. 17 (5): 244–251. doi: 10.1016/j.molmed.2011.01.007. PMC 3092840. PMID 21388883.
- ^ Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, et al. (March 2005). "The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility". Science. 307 (5714): 1434–1440. Bibcode: 2005Sci...307.1434G. doi: 10.1126/science.1101160. PMID 15637236. S2CID 8815153.
- ^ a b Liu S, Yao L, Ding D, Zhu H (December 2010). "CCL3L1 copy number variation and susceptibility to HIV-1 infection: a meta-analysis". PLOS ONE. 5 (12): e15778. Bibcode: 2010PLoSO...515778L. doi: 10.1371/journal.pone.0015778. PMC 3012711. PMID 21209899.
- ^ Dube S, Qin J, Ramakrishnan R (August 2008). "Mathematical analysis of copy number variation in a DNA sample using digital PCR on a nanofluidic device". PLOS ONE. 3 (8): e2876. Bibcode: 2008PLoSO...3.2876D. doi: 10.1371/journal.pone.0002876. PMC 2483940. PMID 18682853.
- ^ Hughesman CB, Lu XJ, Liu KY, Zhu Y, Towle RM, Haynes C, Poh CF (September 2017). "Detection of clinically relevant copy number alterations in oral cancer progression using multiplexed droplet digital PCR". Scientific Reports. 7 (1): 11855. Bibcode: 2017NatSR...711855H. doi: 10.1038/s41598-017-11201-4. PMC 5605662. PMID 28928368.
- ^ a b Usher CL, Handsaker RE, Esko T, Tuke MA, Weedon MN, Hastie AR, et al. (August 2015). "Structural forms of the human amylase locus and their relationships to SNPs, haplotypes and obesity". Nature Genetics. 47 (8): 921–925. doi: 10.1038/ng.3340. PMC 4712930. PMID 26098870.
- ^ Aldhous MC, Abu Bakar S, Prescott NJ, Palla R, Soo K, Mansfield JC, et al. (December 2010). "Measurement methods and accuracy in copy number variation: failure to replicate associations of beta-defensin copy number with Crohn's disease". Human Molecular Genetics. 19 (24): 4930–4938. doi: 10.1093/hmg/ddq411. PMC 2989891. PMID 20858604.
- ^ Pinheiro L, Emslie KR (2018). "Basic Concepts and Validation of Digital PCR Measurements". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 11–24. doi: 10.1007/978-1-4939-7778-9_2. ISBN 978-1-4939-7776-5. PMID 29717435.
- ^ Handsaker RE, Van Doren V, Berman JR, Genovese G, Kashin S, Boettger LM, McCarroll SA (March 2015). "Large multiallelic copy number variations in humans". Nature Genetics. 47 (3): 296–303. doi: 10.1038/ng.3200. PMC 4405206. PMID 25621458.
- ^ Garcia-Murillas I, Turner NC (2018). "Assessing HER2 Amplification in Plasma cfDNA". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 161–172. doi: 10.1007/978-1-4939-7778-9_10. ISBN 978-1-4939-7776-5. PMID 29717443.
- ^ Christgen M, van Luttikhuizen JL, Raap M, Braubach P, Schmidt L, Jonigk D, et al. (December 2016). "Precise ERBB2 copy number assessment in breast cancer by means of molecular inversion probe array analysis". Oncotarget. 7 (50): 82733–82740. doi: 10.18632/oncotarget.12421. PMC 5347728. PMID 27716627.
- ^ Borley A, Mercer T, Morgan M, Dutton P, Barrett-Lee P, Brunelli M, Jasani B (April 2014). "Impact of HER2 copy number in IHC2+/FISH-amplified breast cancer on outcome of adjuvant trastuzumab treatment in a large UK cancer network". British Journal of Cancer. 110 (8): 2139–2143. doi: 10.1038/bjc.2014.147. PMC 3992505. PMID 24691421.
- ^ Cao Y, Raith MR, Griffith JF (March 2015). "Droplet digital PCR for simultaneous quantification of general and human-associated fecal indicators for water quality assessment". Water Research. 70: 337–349. Bibcode: 2015WatRe..70..337C. doi: 10.1016/j.watres.2014.12.008. PMID 25543243.
- ^ European Society for Medical Oncology (17 Nov 2017). "Study analyzes mutations in cerebrospinal fluid in lung cancer with brain metastases". Retrieved 24 July 2019.
- ^ a b Petrone J (8 June 2017). "Norwegian Team Plans to Debut Digital PCR-Based Urinary Bladder Cancer Test by Year End". Retrieved 24 July 2019.
- ^ Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, Jiwa NM, de Boer M, Leguit RJ, et al. (April 2018). "The use of droplet digital PCR in liquid biopsies: A highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid". Hematological Oncology. 36 (2): 429–435. doi: 10.1002/hon.2489. PMID 29210102. S2CID 4968214.
- ^ a b Paxton A (October 2017). "Revived hopes, fresh challenges with liquid biopsy". Retrieved 24 July 2019.
- ^ Bhadra K, Mellert H, Pestano G (5 Jun 2017). "Adoption of Liquid Biopsy Tests for NSCLC". Retrieved 24 July 2019.
- ^ Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, et al. (August 2016). "Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer". JAMA Oncology. 2 (8): 1014–1022. doi: 10.1001/jamaoncol.2016.0173. PMC 4982795. PMID 27055085.
- ^ Olsson E, Winter C, George A, Chen Y, Howlin J, Tang MH, et al. (August 2015). "Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease". EMBO Molecular Medicine. 7 (8): 1034–1047. doi: 10.15252/emmm.201404913. PMC 4551342. PMID 25987569.
- ^ Carpinetti P, Donnard E, Bettoni F, Asprino P, Koyama F, Rozanski A, et al. (November 2015). "The use of personalized biomarkers and liquid biopsies to monitor treatment response and disease recurrence in locally advanced rectal cancer after neoadjuvant chemoradiation". Oncotarget. 6 (35): 38360–38371. doi: 10.18632/oncotarget.5256. PMC 4742005. PMID 26451609.
- ^ Reinert T, Schøler LV, Thomsen R, Tobiasen H, Vang S, Nordentoft I, et al. (April 2016). "Analysis of circulating tumour DNA to monitor disease burden following colorectal cancer surgery". Gut. 65 (4): 625–634. doi: 10.1136/gutjnl-2014-308859. PMID 25654990.
- ^ Pereira E, Camacho-Vanegas O, Anand S, Sebra R, Catalina Camacho S, Garnar-Wortzel L, et al. (2015). "Personalized Circulating Tumor DNA Biomarkers Dynamically Predict Treatment Response and Survival In Gynecologic Cancers". PLOS ONE. 10 (12): e0145754. Bibcode: 2015PLoSO..1045754P. doi: 10.1371/journal.pone.0145754. PMC 4696808. PMID 26717006.
- ^ Dahmcke CM, Steven KE, Larsen LK, Poulsen AL, Abdul-Al A, Dahl C, Guldberg P (December 2016). "A Prospective Blinded Evaluation of Urine-DNA Testing for Detection of Urothelial Bladder Carcinoma in Patients with Gross Hematuria". European Urology. 70 (6): 916–919. doi: 10.1016/j.eururo.2016.06.035. PMID 27417036.
- ^ a b c Lindner L, Cayrou P, Jacquot S, Birling MC, Herault Y, Pavlovic G (July 2021). "Reliable and robust droplet digital PCR (ddPCR) and RT-ddPCR protocols for mouse studies". Methods. 191: 95–106. doi: 10.1016/j.ymeth.2020.07.004. PMID 32721466. S2CID 220851187.
- ^ Taylor SC, Carbonneau J, Shelton DN, Boivin G (November 2015). "Optimization of Droplet Digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: Clinical implications for quantification of Oseltamivir-resistant subpopulations". Journal of Virological Methods. 224: 58–66. doi: 10.1016/j.jviromet.2015.08.014. PMID 26315318.
- ^ Whale AS, Huggett JF, Tzonev S (December 2016). "Fundamentals of multiplexing with digital PCR". Biomolecular Detection and Quantification. 10: 15–23. doi: 10.1016/j.bdq.2016.05.002. PMC 5154634. PMID 27990345.
- ^ Sun B, Tao L, Zheng YL (June 2014). "Simultaneous quantification of alternatively spliced transcripts in a single droplet digital PCR reaction". BioTechniques. 56 (6): 319–325. doi: 10.2144/000114179. PMID 24924392.
- ^ Valencia CA, Rhodenizer D, Bhide S, Chin E, Littlejohn MR, Keong LM, et al. (2012). "Assessment of target enrichment platforms using massively parallel sequencing for the mutation detection for congenital muscular dystrophy". The Journal of Molecular Diagnostics. 14 (3): 233–246. doi: 10.1016/j.jmoldx.2012.01.009. PMC 3349841. PMID 22426012.
- ^ Philippe J, Derhourhi M, Durand E, Vaillant E, Dechaume A, Rabearivelo I, et al. (2015). "What Is the Best NGS Enrichment Method for the Molecular Diagnosis of Monogenic Diabetes and Obesity?". PLOS ONE. 10 (11): e0143373. Bibcode: 2015PLoSO..1043373P. doi: 10.1371/journal.pone.0143373. PMC 4657897. PMID 26599467.
- ^ Ouellet E, Foley JH, Conway EM, Haynes C (August 2015). "Hi-Fi SELEX: A High-Fidelity Digital-PCR Based Therapeutic Aptamer Discovery Platform". Biotechnology and Bioengineering. 112 (8): 1506–1522. doi: 10.1002/bit.25581. PMID 25727321. S2CID 39450798.
- ^ Ludlow AT, Shelton D, Wright WE, Shay JW (2018). "DdTRAP: A Method for Sensitive and Precise Quantification of Telomerase Activity". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 513–529. doi: 10.1007/978-1-4939-7778-9_29. ISBN 978-1-4939-7776-5. PMC 6046637. PMID 29717462.
- ^ Sayed ME, Slusher AL, Ludlow AT (May 2019). "Droplet Digital TRAP (ddTRAP): Adaptation of the Telomere Repeat Amplification Protocol to Droplet Digital Polymerase Chain Reaction". Journal of Visualized Experiments (147). doi: 10.3791/59550. PMID 31107456. S2CID 155519448.
- ^ Stein RA (1 July 2019). "Single-Cell Sequencing Sifts through Multiple Omics". Retrieved 1 August 2019.
- ^ Wood-Bouwens CM, Ji HP (2018). "Single Color Multiplexed DDPCR Copy Number Measurements and Single Nucleotide Variant Genotyping". Digital PCR. Methods in Molecular Biology. Vol. 1768. pp. 323–333. doi: 10.1007/978-1-4939-7778-9_18. ISBN 978-1-4939-7776-5. PMID 29717451.
- ^ Low, H., Chan, SJ., Soo, GH. et al. Clarity™ digital PCR system: a novel platform for absolute quantification of nucleic acids. Anal Bioanal Chem 409, 1869–1875 (2017). https://doi.org/10.1007/s00216-016-0131-7
- ^ Saiki RK, Gelfand DH, Stoffel S, Scharf SJ, Higuchi R, Horn GT, et al. (January 1988). "Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase". Science. 239 (4839): 487–491. Bibcode: 1988Sci...239..487S. doi: 10.1126/science.239.4839.487. PMID 2448875.
- ^ Morley AA (September 2014). "Digital PCR: A brief history". Biomolecular Detection and Quantification. 1 (1): 1–2. doi: 10.1016/j.bdq.2014.06.001. PMC 5129430. PMID 27920991.
- ^ Rutsaert S, Bosman K, Trypsteen W, Nijhuis M, Vandekerckhove L (January 2018). "Digital PCR as a tool to measure HIV persistence". Retrovirology. 15 (1): 16. doi: 10.1186/s12977-018-0399-0. PMC 5789538. PMID 29378600.
- ^ a b Perkel J (11 April 2014). "The digital PCR revolution". Retrieved 22 July 2019.
- ^ Pohl G, Shih I (January 2004). "Principle and applications of digital PCR". Expert Review of Molecular Diagnostics. 4 (1): 41–47. doi: 10.1586/14737159.4.1.41. PMID 14711348. S2CID 28271641.
- ^ Dressman D, Yan H, Traverso G, Kinzler KW, Vogelstein B (July 2003). "Transforming single DNA molecules into fluorescent magnetic particles for detection and enumeration of genetic variations". Proceedings of the National Academy of Sciences of the United States of America. 100 (15): 8817–8822. Bibcode: 2003PNAS..100.8817D. doi: 10.1073/pnas.1133470100. PMC 166396. PMID 12857956.
- ^ Diehl F, Li M, He Y, Kinzler KW, Vogelstein B, Dressman D (July 2006). "BEAMing: single-molecule PCR on microparticles in water-in-oil emulsions". Nature Methods. 3 (7): 551–559. doi: 10.1038/nmeth898. PMID 16791214. S2CID 7059151.
- ^ Butkus B (8 July 2010). "Digital PCR Space Heating Up as Life Science Tool Vendors Begin Staking Claims". Retrieved 22 July 2019.
- ^ Ramakrishnan R, Qin J, Jones RC, Weaver LS (2013). "Integrated Fluidic Circuits (IFCs) for Digital PCR". Microfluidic Diagnostics. Methods in Molecular Biology. Vol. 949. pp. 423–431. doi: 10.1007/978-1-62703-134-9_27. ISBN 978-1-62703-133-2. PMID 23329458.
- ^ Butkus B (29 Mar 2012). "RainDance Launches Digital PCR Platform; Claims Sensitivity, Operating Cost Superiority". Retrieved 22 July 2019.
- ^ "'Liquid biopsy' blood test detects genetic mutations in common form of lung cancer". 7 Apr 2016. Retrieved 22 July 2019.
- ^ "Korea's BioCore First to Commercialize NIPT Based on Digital PCR". 2 Mar 2018. Retrieved 22 July 2019.
- ^ "Bio-Rad Gets First CE Mark on Clinical ddPCR Test". 5 Dec 2017. Retrieved 22 Jul 2019.