| |
| |
| Names | |
|---|---|
| Other names
Bis(Pentamethylcyclopentadienyl)titanium dichloride
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.149.726 |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H30Cl2Ti | |
| Molar mass | 389.23 g·mol−1 |
| Appearance | red solid |
| Density | 1.32 g/cm3 |
| Melting point | 190 °C (374 °F; 463 K) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
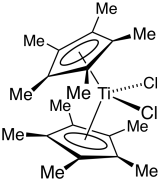
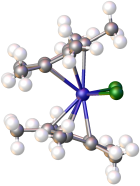
Decamethyltitanocene dichloride is an organotitanium compound with the formula Cp*2TiCl2 (where Cp* is C5(CH3)5, derived from pentamethylcyclopentadiene). It is a red solid that is soluble in nonpolar organic solvents. The complex has been the subject of extensive research. It is a precursor to many organotitanium complexes. The complex is related to titanocene dichloride, which lacks the methyl groups.
Synthesis and reactions
The complex is prepared by the reaction of titanium tetrachloride with LiCp*. An intermediate in this synthesis is (pentamethylcyclopentadienyl)titanium trichloride.
Reduction of Cp*2TiCl2 in the presence of ethylene gives the adduct Cp*2Ti(C2H4). [1] The analogous Cp compound has not been prepared, a contrast that highlights the advantages of the Cp* ligand. This pentamethylcyclopentadienyl (Cp*) species undergoes many reactions such as cycloadditions of alkynes. [2]
The dicarbonyl complex Cp*2Ti(CO)2 is prepared by reduction of Cp*2TiCl2 in the presence of carbon monoxide. [3]
Further reading
- Rosenthal, U.; et al. (2000). "What Do Titano- and Zirconocenes Do with Diynes and Polyynes?". Chemical Reviews. 33 (2): 119–129. doi: 10.1021/ar9900109. PMID 10673320.
References
- ^ Cohen, Steven A.; Auburn, Pamela R.; Bercaw, John E. (1983). "Structure and Reactivity of Bis(pentamethylcyclopentadienyl)(ethylene)titanium(II), a Simple Olefin Adduct of Titanium". Journal of the American Chemical Society. 105 (5): 1136–1143. doi: 10.1021/ja00343a012.
- ^ *Buchwald, S. L.; Nielsen, R. B. (1988). "Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes". Chemical Reviews. 88 (7): 1047–1058. doi: 10.1021/cr00089a004.
- ^ Sikora, David J.; Moriarty, Kevin J.; Rausch, Marvin D. (1990). Dicarbonylbis(η5 -Cyclopentadienyl) Complexes of Titanium, Zirconium, and Hafnium. Inorganic Syntheses. Vol. 28. pp. 248–257. doi: 10.1002/9780470132593.ch64. ISBN 9780470132593.

| |

| |
| Names | |
|---|---|
| Other names
Bis(Pentamethylcyclopentadienyl)titanium dichloride
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.149.726 |
PubChem
CID
|
|
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C20H30Cl2Ti | |
| Molar mass | 389.23 g·mol−1 |
| Appearance | red solid |
| Density | 1.32 g/cm3 |
| Melting point | 190 °C (374 °F; 463 K) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Decamethyltitanocene dichloride is an organotitanium compound with the formula Cp*2TiCl2 (where Cp* is C5(CH3)5, derived from pentamethylcyclopentadiene). It is a red solid that is soluble in nonpolar organic solvents. The complex has been the subject of extensive research. It is a precursor to many organotitanium complexes. The complex is related to titanocene dichloride, which lacks the methyl groups.
Synthesis and reactions
The complex is prepared by the reaction of titanium tetrachloride with LiCp*. An intermediate in this synthesis is (pentamethylcyclopentadienyl)titanium trichloride.
Reduction of Cp*2TiCl2 in the presence of ethylene gives the adduct Cp*2Ti(C2H4). [1] The analogous Cp compound has not been prepared, a contrast that highlights the advantages of the Cp* ligand. This pentamethylcyclopentadienyl (Cp*) species undergoes many reactions such as cycloadditions of alkynes. [2]
The dicarbonyl complex Cp*2Ti(CO)2 is prepared by reduction of Cp*2TiCl2 in the presence of carbon monoxide. [3]
Further reading
- Rosenthal, U.; et al. (2000). "What Do Titano- and Zirconocenes Do with Diynes and Polyynes?". Chemical Reviews. 33 (2): 119–129. doi: 10.1021/ar9900109. PMID 10673320.
References
- ^ Cohen, Steven A.; Auburn, Pamela R.; Bercaw, John E. (1983). "Structure and Reactivity of Bis(pentamethylcyclopentadienyl)(ethylene)titanium(II), a Simple Olefin Adduct of Titanium". Journal of the American Chemical Society. 105 (5): 1136–1143. doi: 10.1021/ja00343a012.
- ^ *Buchwald, S. L.; Nielsen, R. B. (1988). "Group 4 Metal Complexes of Benzynes, Cycloalkynes, Acyclic Alkynes, and Alkenes". Chemical Reviews. 88 (7): 1047–1058. doi: 10.1021/cr00089a004.
- ^ Sikora, David J.; Moriarty, Kevin J.; Rausch, Marvin D. (1990). Dicarbonylbis(η5 -Cyclopentadienyl) Complexes of Titanium, Zirconium, and Hafnium. Inorganic Syntheses. Vol. 28. pp. 248–257. doi: 10.1002/9780470132593.ch64. ISBN 9780470132593.