| |
| Names | |
|---|---|
|
Preferred IUPAC name
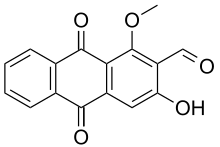
3-Hydroxy-1-methoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde | |
| Other names
3-Hydroxy-1-methoxyanthraquinone-2-aldehyde
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.625 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C16H10O5 | |
| Molar mass | 282.251 g·mol−1 |
| Density | 1.461 g/mL |
| Boiling point | 532 °C (990 °F; 805 K) |
| Related compounds | |
Related arylformaldehydes
|
Gossypol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Damnacanthal is an anthraquinone isolated from the root of Morinda citrifolia, using water [1] or organic solvents. [2]
Pharmacology
In a 1995 in vitro study, damnacanthal was found to act as a potent and selective inhibitor of p56lck tyrosine kinase. [3]
References
- ^ Anekpankul T, Goto M, Sasaki M, et al. (July 2007). "Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water". Separation and Purification Technology. 55 (3): 343–349. doi: 10.1016/j.seppur.2007.01.004.
- ^ Okusada K, Nakamoto K, Nishida M, Fujita-Hamabe W, Kamiya K, Mizushina Y, Satake T, Tokuyama S (2011). "The antinociceptive and anti-inflammatory action of the CHCl3-soluble phase and its main active component, damnacanthal, isolated from the root of Morinda citrifolia". Biol Pharm Bull. 34 (1): 103–7. doi: 10.1248/bpb.34.103. PMID 21212526.
- ^ Faltynek CR, Schroeder J, Mauvais P, et al. (September 1995). "Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity". Biochemistry. 34 (38): 12404–10. doi: 10.1021/bi00038a038. PMID 7547985.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
3-Hydroxy-1-methoxy-9,10-dioxo-9,10-dihydroanthracene-2-carbaldehyde | |
| Other names
3-Hydroxy-1-methoxyanthraquinone-2-aldehyde
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.208.625 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C16H10O5 | |
| Molar mass | 282.251 g·mol−1 |
| Density | 1.461 g/mL |
| Boiling point | 532 °C (990 °F; 805 K) |
| Related compounds | |
Related arylformaldehydes
|
Gossypol |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Damnacanthal is an anthraquinone isolated from the root of Morinda citrifolia, using water [1] or organic solvents. [2]
Pharmacology
In a 1995 in vitro study, damnacanthal was found to act as a potent and selective inhibitor of p56lck tyrosine kinase. [3]
References
- ^ Anekpankul T, Goto M, Sasaki M, et al. (July 2007). "Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water". Separation and Purification Technology. 55 (3): 343–349. doi: 10.1016/j.seppur.2007.01.004.
- ^ Okusada K, Nakamoto K, Nishida M, Fujita-Hamabe W, Kamiya K, Mizushina Y, Satake T, Tokuyama S (2011). "The antinociceptive and anti-inflammatory action of the CHCl3-soluble phase and its main active component, damnacanthal, isolated from the root of Morinda citrifolia". Biol Pharm Bull. 34 (1): 103–7. doi: 10.1248/bpb.34.103. PMID 21212526.
- ^ Faltynek CR, Schroeder J, Mauvais P, et al. (September 1995). "Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity". Biochemistry. 34 (38): 12404–10. doi: 10.1021/bi00038a038. PMID 7547985.