Chloropyridines are a group of aryl chlorides consisting of a pyridine ring with chlorine atoms as substituents.
This may refer to:
Production
Direct halogenation of pyridine with chlorine gas above 270 °C gives a mixture of 2-chloropyridine and 2,6-dichloropyridine. [1]
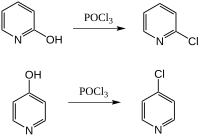
2- and 4-chloropyridine are prepared from the corresponding pyridinols using phosphoryl chloride: [1]
Uses
Chloropyridines are important intermediates to pharmaceuticals and agrochemicals. [1] A major use of 2-chloropyridine is the production of production of the fungicide pyrithione. Reaction of 4-chloropyridine with thioglycolic acid gives pyridylmercaptoacetic acid, a step in the production of cephalosporin antibiotics.
See also
References
- ^ a b c Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a22_399. ISBN 978-3527306732.
Chloropyridines are a group of aryl chlorides consisting of a pyridine ring with chlorine atoms as substituents.
This may refer to:
Production
Direct halogenation of pyridine with chlorine gas above 270 °C gives a mixture of 2-chloropyridine and 2,6-dichloropyridine. [1]
2- and 4-chloropyridine are prepared from the corresponding pyridinols using phosphoryl chloride: [1]
Uses
Chloropyridines are important intermediates to pharmaceuticals and agrochemicals. [1] A major use of 2-chloropyridine is the production of production of the fungicide pyrithione. Reaction of 4-chloropyridine with thioglycolic acid gives pyridylmercaptoacetic acid, a step in the production of cephalosporin antibiotics.
See also
References
- ^ a b c Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2000). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi: 10.1002/14356007.a22_399. ISBN 978-3527306732.