| |
| Names | |
|---|---|
|
Preferred IUPAC name
Di(propan-2-yl)phosphinous chloride | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.609 |
| EC Number |
|
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C6H14ClP | |
| Molar mass | 152.60 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.959 g/mL at 25 °C |
| Boiling point | 46-47 °C (10 mm of Hg) |
| Reacts | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, reacts with water to release HCl |
| GHS labelling: [1] | |


| |
| Danger | |
| H225, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
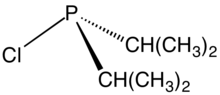
Chlorodiisopropylphosphine is an organophosphorus compound with the formula [(CH3)2CH]2PCl. It is a colorless liquid that reacts with water and oxygen. The compound is used to prepare tertiary phosphines and phosphinite ligands.
Synthesis and reactions
The compound is prepared by treating phosphorus trichloride with the Grignard reagent isopropylmagnesium chloride: [2]
- PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2
Relative to the reaction of less hindered Grignard reagents with PCl3, this reaction affords a superior yield of the monochloro derivative.
Chlorodiisopropylphosphine reacts with Grignard reagents and organolithium compounds to give phosphines:
- [(CH3)2CH]2PCl + RM → [(CH3)2CH]2PR + MCl
Chlorodiisopropylphosphine reacts with alcohols and phenols to give phosphinites, this reaction typically is conducted in the presence of a base:
- [(CH3)2CH]2PCl + ROH → [(CH3)2CH]2POR + HCl
Phosphinites are versatile ligands. [3]
References
- ^ "Chlorodiisopropylphosphine". pubchem.ncbi.nlm.nih.gov.
- ^ W. Voskuil; J. F. Arens (1968). "Chlorodiisopropylphosphine". Org. Synth. 48: 47. doi: 10.15227/orgsyn.048.0047.
- ^ for example: Pandarus, V., Zargarian, D., "New Pincer-Type Diphosphinito (POCOP) Complexes of Nickel", Organometallics 2007, volume 26, 4321. doi: 10.1021/om700400x

| |
| Names | |
|---|---|
|
Preferred IUPAC name
Di(propan-2-yl)phosphinous chloride | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.157.609 |
| EC Number |
|
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C6H14ClP | |
| Molar mass | 152.60 g·mol−1 |
| Appearance | colorless liquid |
| Density | 0.959 g/mL at 25 °C |
| Boiling point | 46-47 °C (10 mm of Hg) |
| Reacts | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Toxic, reacts with water to release HCl |
| GHS labelling: [1] | |


| |
| Danger | |
| H225, H314 | |
| P210, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P403+P235, P405, P501 | |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Chlorodiisopropylphosphine is an organophosphorus compound with the formula [(CH3)2CH]2PCl. It is a colorless liquid that reacts with water and oxygen. The compound is used to prepare tertiary phosphines and phosphinite ligands.
Synthesis and reactions
The compound is prepared by treating phosphorus trichloride with the Grignard reagent isopropylmagnesium chloride: [2]
- PCl3 + 2 (CH3)2CHMgCl → [(CH3)2CH]2PCl + 2 MgCl2
Relative to the reaction of less hindered Grignard reagents with PCl3, this reaction affords a superior yield of the monochloro derivative.
Chlorodiisopropylphosphine reacts with Grignard reagents and organolithium compounds to give phosphines:
- [(CH3)2CH]2PCl + RM → [(CH3)2CH]2PR + MCl
Chlorodiisopropylphosphine reacts with alcohols and phenols to give phosphinites, this reaction typically is conducted in the presence of a base:
- [(CH3)2CH]2PCl + ROH → [(CH3)2CH]2POR + HCl
Phosphinites are versatile ligands. [3]
References
- ^ "Chlorodiisopropylphosphine". pubchem.ncbi.nlm.nih.gov.
- ^ W. Voskuil; J. F. Arens (1968). "Chlorodiisopropylphosphine". Org. Synth. 48: 47. doi: 10.15227/orgsyn.048.0047.
- ^ for example: Pandarus, V., Zargarian, D., "New Pincer-Type Diphosphinito (POCOP) Complexes of Nickel", Organometallics 2007, volume 26, 4321. doi: 10.1021/om700400x