Charles Brenner | |
|---|---|
| Born | October 30, 1961 |
| Nationality | American |
| Alma mater |
Wesleyan University (
BA) Stanford University ( PhD) Brandeis University |
| Known for | Discovery and characterization of nicotinamide riboside as a vitamin |
| Awards | Fellow of the American Association for the Advancement of Science |
| Scientific career | |
| Fields | Enzymology Metabolism |
| Institutions |
City of Hope National Medical Center University of Iowa Dartmouth Medical School Thomas Jefferson University |
| Thesis | Specificity and Activity of the Kex2 Protease: From Yeast Genetics to Enzyme Kinetics (1993) |
| Doctoral advisor | Robert S. Fuller |
| Other academic advisors |
Gregory A. Petsko Dagmar Ringe |
| Notable students | Peter A. Belenky, Samuel A.J. Trammell |
| Website |
brennerlab |
Charles Brenner (born October 30, 1961) is the inaugural Alfred E Mann Family Foundation Chair of the Department of Diabetes & Cancer Metabolism at the Beckman Research Institute of the City of Hope National Medical Center. Brenner previously held the Roy J. Carver Chair in Biochemistry and was head of biochemistry at the University of Iowa. [1] [2]
Brenner is a major contributor in the field of nicotinamide adenine dinucleotide (NAD) metabolism and has developed targeted, quantitative methods for NAD metabolomics. [3] Brenner discovered eukaryotic nicotinamide riboside (NR) kinase and nucleosidase pathways to NAD. [4] [5] Brenner's work includes the first human trial of NR, which demonstrated safe oral availability as an NAD+ precursor. [6] [4] He has characterized ways in which NAD is disrupted by diseases and metabolic stress. [2]
Brenner graduated from Wesleyan University with a bachelor's degree in biology in 1983. After working for the biotechnology companies Chiron Corporation and DNAX Research Institute, Brenner attended graduate school at Stanford University School of Medicine. At Stanford he worked with Robert S. Fuller, receiving his Ph.D. in Cancer Biology in 1993. Brenner conducted post-doctoral research at Brandeis University with Gregory Petsko and Dagmar Ringe. [7] [8]
Brenner then joined the faculty at Thomas Jefferson University, where he worked from 1996-2003, becoming Director of the Structural Biology & Bioinformatics Program in 2000. He moved to Dartmouth Medical School in 2003, serving as Associate Director for Basic Sciences at Norris Cotton Cancer Center (now named Dartmouth Cancer Center) from 2003-2009. In 2009 he joined the University of Iowa (UI) as Professor and Departmental Executive Officer (DEO) of Biochemistry. In 2010 he became the Roy J. Carver Chair of Biochemistry at UI, holding that position until 2020. [9] [2] [10]
In 2020, Brenner joined City of Hope National Medical Center in Duarte, California as the inaugural Alfred E Mann Family Foundation Chair in Diabetes and Cancer Metabolism. City of Hope created the position and the associated Department of Diabetes & Cancer Metabolism to focus on underlying metabolism and the intersection of metabolic disturbances with diseases such as cancer and diabetes. [2] [1]
Brenner has been funded by agencies including the March of Dimes, [11] the Burroughs Wellcome Fund, [11] the Beckman Foundation, [12] the Lung Cancer Research Foundation, [13] the Bill & Melinda Gates Foundation, [14] the Leukemia & Lymphoma Society, the National Science Foundation. and the National Institutes of Health. [15]
Brenner has made multiple contributions to molecular biology and biochemistry, beginning with purification and characterization of the Kex2 proprotein convertase at Stanford. [16] [17] Significant research projects include the role of Ap3A bindings in the function of the FHIT tumor suppressor gene, [18] characterization and inhibition of DNA methylation, [19] [20] and discovery of new steps in nicotinamide adenine dinucleotide (NAD) metabolism. [21]
Notably, the Brenner laboratory discovered that eukaryotes use nicotinamide riboside (NR) to make NAD+. Bieganowski and Brenner (2004) found that NR is converted to NAD+ through the action of nicotinamide ribose kinases including Nrk1 (yeast and human) and Nrk2 (human). Belenky et al (Cell, 2007) reported another pathway which turns NR into NAM through the action of nucleosidases Urh1/Pnp1/Meu1 and is Nrk1 independent. [4] [22] [6] [23]
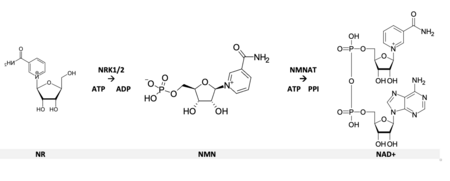
Brenner has developed targeted, quantitative analysis of the NAD+ metabolome [3] [24] and made fundamental contributions to NAD metabolism including discovery of nicotinic acid riboside-dependent NAD synthesis, [25] elucidating the mechanism of synthesis of nicotinic acid adenine dinucleotide phosphate, [26] and discovering multiple conditions in which NAD metabolism is dysregulated in disease. [27] [28] [29] [30] [31] [32]
Brenner is active in translating NR technologies to treat and prevent human conditions that disturb the NAD system including cancer [29] diabetic and chemotherapeutic peripheral neuropathy, [33] [34] heart failure, [28] central brain injury, [30] inflammation, [31] mitochondrial myopathy [32] pellagra, and infections [27] such as coronavirus infection [6] [35] Brenner's work included the first human trial of NR in 2016, which demonstrated safe oral availability as an NAD+ precursor. [6] [4] Though Brenner was the first to show that NR increases SIR2 activity, improves gene silencing, and can extend yeast lifespan, [6] [36] his work has not emphasized sirtuins or nonspecific anti-aging claims and instead emphasizes how NR repairs metabolic stresses that dysregulate NAD+ [28] [30] and NADPH. [6] [37]
| External videos | |
|---|---|
Examining rodents and their offspring, Brenner has showed that rodent postpartum mothers are under severe metabolic stress to their NAD system. Supplementing rodent mothers with NR increases maternal weight loss, advances juvenile development and provides long lasting neurodevelopmental advantages into adulthood. [38] [39] [14]
Brenner is an author of more than 200 peer-reviewed publications. [40] He was the senior editor of the 2004 book, Oncogenomics: Molecular Approaches to Cancer. [41]
Brenner is both cautious and critical of research that promotes claims of anti-aging and longevity. [42] [43] [44] After writing a favorable review of Steven Austad's book Methuselah's Zoo, [45] he reviewed Lifespan: Why We Age – and Why We Don't Have To by David A. Sinclair, summarizing it as "an influential source of misinformation on longevity, featuring counterfactual claims about longevity genes being conserved between yeast and humans, the existence of supposed activators of these genes, and claimed successful age reversal in mice based on partial reprogramming." [46] Brenner published a major review of sirtuins in 2022 entitled "Sirtuins are not conserved longevity genes". [47]
| External videos | |
|---|---|
In 2012, Brenner and Dagmar Ringe developed pre-medical curriculum recommendations that would be consistent with a revised Medical College Admission Test (MCAT), following a request from the President of the American Society for Biochemistry and Molecular Biology, Suzanne Pfeffer. [48] [49] The recommendations, which include development of inorganic, organic and biochemistry coursework that is more geared toward the chemistry of bioorganic functional groups, have been further refined in academic journals. Brenner's contribution to this area was recognized by the 2016 ASBMB Award for Exemplary Contributions to Education. [50]
Brenner is a former member of the Scientific Advisory Board of Sirtris Pharmaceuticals. [51] He was a co-founder of ProHeathspan prior to its acquisition by ChromaDex, and serves as member of the scientific advisory board and chief scientific advisor to ChromaDex. [7] [52]
- 2020, Mary Swartz Rose Senior Investigator Award, American Society for Nutrition [53]
- 2016, ASBMB Award for Exemplary Contributions to Education, American Society for Biochemistry and Molecular Biology [50]
- 2013, Fellow, American Association for the Advancement of Science [54]
- 2007, William E.M. Lands Lectureship, University of Michigan Medical School [55]
- 1998-2001, New Investigator in the Pharmacological Sciences, Burroughs Wellcome Fund [11]
- 1998-2000, Basil O'Connor Scholar, March of Dimes Birth Defects Foundation [11]
- 1998-2000, Beckman Young Investigators Award, Arnold and Mabel Beckman Foundation [12]
- Brenner, C (2022-09-22). "Sirtuins are not conserved longevity genes". Life Metabolism (2): 122–133. doi: 10.1093/lifemeta/loac025. ISSN 2755-0230. PMC 10081735. PMID 37035412.
- Brenner, C (January 2022). "Viral infection as an NAD+ battlefield". Nature Metabolism. 4 (1): 2–3. doi: 10.1038/s42255-021-00507-3. ISSN 2522-5812. PMC 10155260. PMID 34980922. S2CID 245654307.
- Heer, CD; Sanderson, DJ; Voth, LS; Alhammad, YMO; Schmidt, MS; Trammell, SAJ; Perlman, S; Cohen, MS; Fehr, AR; Brenner, C (2020-10-13). "Coronavirus infection and PARP expression dysregulate the NAD Metabolome: an actionable component of innate immunity". Journal of Biological Chemistry. 295 (52): 17986–17996. doi: 10.1074/jbc.RA120.015138. PMC 7834058. PMID 33051211.
- Vaur, P; Brugg, B; Mericskay, M; Li, Z; Schmidt, M S.; Vivien, D; Orset, C; Jacotot, E; Brenner, C (December 2017). "Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration". FASEB Journal. 31 (12): 5440–5452. doi: 10.1096/fj.201700221RR. ISSN 1530-6860. PMID 28842432.
- Trammell, SAJ; Schmidt, MS; Weidemann, BJ; Redpath, P; Jaksch, F; Dellinger, RW; Li, Z; Abel, ED; Migaud, ME; Brenner, C (10 October 2016). "Nicotinamide riboside is uniquely and orally bioavailable in mice and humans". Nature Communications. 7 (1): 12948. Bibcode: 2016NatCo...712948T. doi: 10.1038/ncomms12948. PMC 5062546. PMID 27721479.
- Trammell, SAJ; Weidemann, BJ; Chadda, A; Yorek, MS; Holmes, A; Coppey, LJ; Obrosov, A; Kardon, RH; Yorek, MA; Brenner, C (2016). "Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice". Scientific Reports. 6: 26933. Bibcode: 2016NatSR...626933T. doi: 10.1038/srep26933. PMC 4882590. PMID 27230286.
- Wu, B-K; Brenner, C (2014). "Suppression of TET1-Dependent DNA Demethylation Is Essential for KRAS-Mediated Transformation". Cell Reports. 9 (5): 1827–1840. doi: 10.1016/j.celrep.2014.10.063. PMC 4268240. PMID 25466250.
- Fagan, RL; Cryderman, DE; Kopelovich, L; Wallrath, LL; Brenner, C (2013). "Laccaic Acid A Is a Direct, DNA-competitive Inhibitor of DNA Methyltransferase 1". J. Biol. Chem. 288 (33): 23858–23867. doi: 10.1074/jbc.M113.480517. PMC 3745332. PMID 23839987.
- Brenner, C (2013). "Changes in Chemistry and Biochemistry Education: Creative Responses to MCAT Revisions in the Age of the Genome". Biochemistry and Molecular Biology Education. 41 (1): 1–4. doi: 10.1002/bmb.20653. PMID 23281187. S2CID 4659938.
- Brenner, C (2013). "Rethinking Premedical and Health Professional Curricula in Light of MCAT 2015" (PDF). J. Chem. Educ. 90 (7): 807–812. Bibcode: 2013JChEd..90..807B. doi: 10.1021/ed4002738. S2CID 98274150.
- Syeda, F; Fagan, RL; Wean, M; Avvakumov, GV; Walker, JR; Xue, S; Dhe-Paganon, S; Brenner, C (2011). "The Replication Focus Targeting Sequence (RFTS) Domain is a DNA-Competitive Inhibitor of Dnmt1". J. Biol. Chem. 286 (17): 15344–15351. doi: 10.1074/jbc.M110.209882. PMC 3083197. PMID 21389349.
- Bogan, KL; Brenner, C (1 August 2008). "Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD + Precursor Vitamins in Human Nutrition". Annual Review of Nutrition. 28 (1): 115–130. doi: 10.1146/annurev.nutr.28.061807.155443. ISSN 0199-9885. PMID 18429699.
- Tempel, W; Rabeh, WM; Bogan, KL; Belenky, P; Wojcik, M; Seidle, HF; Nedyalkova, L; Yang, T; Sauve, AA; Park, HW; Brenner, C (2007-10-02). "Nicotinamide riboside kinase structures reveal new pathways to NAD+". PLOS Biology. 5 (10): e263. doi: 10.1371/journal.pbio.0050263. ISSN 1545-7885. PMC 1994991. PMID 17914902.
- Robu, ME; Larson, JD; Nasevicius, A; Beiraghi, S; Brenner, C; Farber, SA; Ekker, SC (25 May 2007). "p53 Activation by Knockdown Technologies". PLOS Genetics. 3 (5): e78. doi: 10.1371/journal.pgen.0030078. ISSN 1553-7404. PMC 1877875. PMID 17530925. S2CID 9914091.
- Belenky, P; Bogan, KL; Brenner, C (January 2007). "NAD+ metabolism in health and disease". Trends in Biochemical Sciences. 32 (1): 12–19. doi: 10.1016/j.tibs.2006.11.006. PMID 17161604.
- Belenky, P; Racette, FG; Bogan, KL; McClure, JM; Smith, JS; Brenner, C (4 May 2007). "Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+". Cell. 129 (3): 473–84. doi: 10.1016/j.cell.2007.03.024. PMID 17482543. S2CID 4661723.
- Bieganowski, P; Brenner, C (2004). "Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans". Cell. 117 (4): 495–502. doi: 10.1016/S0092-8674(04)00416-7. PMID 15137942. S2CID 4642295.
- Brenner, C; Duggan, D, eds. (2004). Oncogenomics: Molecular approaches to cancer. Hoboken, N.J.: Wiley-Liss. ISBN 0-471-22592-4.
- Trapasso, F; Krakowiak, A; Cesari, R; Arkles, J; Yendamuri, S; Ishii, H; Vecchione, A; Kuroki, T; Bieganowski, P; Pace, HC; Huebner, K; Croce, CM; Brenner, C; et al. (18 February 2003). "Designed FHIT alleles establish that Fhit-induced apoptosis in cancer cells is limited by substrate binding". Proceedings of the National Academy of Sciences of the United States of America. 100 (4): 1592–7. Bibcode: 2003PNAS..100.1592T. doi: 10.1073/pnas.0437915100. PMC 149877. PMID 12574506.
- Draganescu, A; Hodawadekar, SC; Gee, KR; Brenner, C (2000). "Fhit-Nucleotide Specificity Probed with Novel Fluorescent and Fluorogenic Substrates". J. Biol. Chem. 275 (7): 4555–4560. doi: 10.1074/jbc.275.7.4555. PMC 2556043. PMID 10671479.
- Brenner, C; Fuller, RS (1992). "Structural and Enzymatic Characterization of a Purified Prohormone-Processing Enzyme: Secreted, Soluble Kex2 Protease". Proc. Natl. Acad. Sci. 89 (3): 922–926. Bibcode: 1992PNAS...89..922B. doi: 10.1073/pnas.89.3.922. PMC 48357. PMID 1736307.
- ^ a b "Leading Biochemist Charles Brenner, PhD, Joins City of Hope as Chair of First Department Focused on Diabetes and Cancer Metabolism". OncLive. MJH Life Sciences. Aug 27, 2020. Retrieved 3 February 2023.
- ^ a b c d "Brenner stepping down as chair of Department of Biochemistry | Department of Biochemistry and Molecular Biology". University of Iowa. July 8, 2020. Retrieved 2 February 2023.
- ^ a b "Awards for Regev and Gierasch; new job for Brenner". ASBMB Today. July 20, 2020.
- ^ a b c d Katsyuba, E; Romani, M; Hofer, D; Auwerx, J (January 2020). "NAD(+) homeostasis in health and disease". Nature Metabolism. 2 (1): 9–31. doi: 10.1038/s42255-019-0161-5. ISSN 2522-5812. PMID 32694684. S2CID 214277961. Retrieved 6 February 2023.
- ^ Cercillieux, A; Ciarlo, E; Canto, C (2022-08-02). "Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations". Cellular and Molecular Life Sciences. 79 (8): 463. doi: 10.1007/s00018-022-04499-5. ISSN 1420-9071. PMC 9345839. PMID 35918544.
- ^ a b c d e f Mehmel, M; Jovanović, N; Spitz, U (31 May 2020). "Nicotinamide Riboside-The Current State of Research and Therapeutic Uses". Nutrients. 12 (6): 1616. doi: 10.3390/nu12061616. PMC 7352172. PMID 32486488.
- ^ a b "ChromaDex To Host Key Opinion Leader Webinar on the Transforming Benefits of Nicotinamide Riboside (NR)". BusinessWire. July 7, 2022. Retrieved 3 February 2023.
- ^ "Vital Signs: Investigator Insight". Dartmouth Medicine Magazine. Vol. 28, no. 4. 2004. Retrieved 2 February 2023.
- ^ "Brenner Named Head of Biochemistry at UI Carver College of Medicine". News-releases.uiowa.edu. May 5, 2009. Archived from the original on July 20, 2011. Retrieved December 9, 2010.
- ^ "CV". Brenner Lab. Retrieved 3 February 2023.
- ^ a b c d "Curriculum Vitae | Charles Brenner Laboratory". University of Iowa. Retrieved 2 February 2023.
- ^ a b "Charles Brenner". Arnold and Mabel Beckman Foundation. Archived from the original on 2 August 2018. Retrieved 1 August 2018.
- ^ "2008 - Dartmouth Medical School - Charles Brenner, PhD". Lung Cancer Research Foundation. Retrieved 6 February 2023.
- ^ a b "Humanitarian Grant Awarded for Preclinical Study on the Impact of NAD Precursor Vitamins on Milk Bioactive Production and Brain Development in Rodents". GlobeNewswire News Room. 7 October 2019. Retrieved 6 February 2023.
- ^ "@author Charles Brenner". Grantome. Retrieved 6 February 2023.
- ^ Nakayama, K (1 November 1997). "Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins". The Biochemical Journal. 327 (3): 625–35. doi: 10.1042/bj3270625. PMC 1218878. PMID 9599222. Retrieved 6 February 2023.
- ^ Sreenivas, S; Krishnaiah, SM; Govindappa, N; Basavaraju, Y; Kanojia, K; Mallikarjun, N; Natarajan, J; Chatterjee, A; Sastry, KN (January 2015). "Enhancement in production of recombinant two-chain Insulin Glargine by over-expression of Kex2 protease in Pichia pastoris" (PDF). Applied Microbiology and Biotechnology. 99 (1): 327–36. doi: 10.1007/s00253-014-6052-5. PMID 25239036. S2CID 253777642.
- ^ Herzog, D; Jansen, J; Mißun, M; Diederichs, K; Stengel, F; Marx, A (18 May 2022). "Chemical Proteomics of the Tumor Suppressor Fhit Covalently Bound to the Cofactor Ap(3)A Elucidates Its Inhibitory Action on Translation". Journal of the American Chemical Society. 144 (19): 8613–8623. doi: 10.1021/jacs.2c00815. ISSN 0002-7863. PMC 9121386. PMID 35522782. S2CID 248554359.
- ^ Syeda, F; Fagan, RL; Wean, M; Avvakumov, GV; Walker, JR; Xue, S; Dhe-Paganon S; Brenner, C (2011). "The Replication Focus Targeting Sequence (RFTS) Domain is a DNA-Competitive Inhibitor of Dnmt1". J. Biol. Chem. 286 (17): 15344–15351. doi: 10.1074/jbc.M110.209882. PMC 3083197. PMID 21389349.
- ^ Wu, B-K; Brenner, C (2014). "Suppression of TET1-Dependent DNA Demethylation Is Essential for KRAS-Mediated Transformation". Cell Reports. 9 (5): 1827–1840. doi: 10.1016/j.celrep.2014.10.063. PMC 4268240. PMID 25466250.
- ^ Elhassan, YS; Philp, AA; Lavery, GG (1 July 2017). "Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule". Journal of the Endocrine Society. 1 (7): 816–835. doi: 10.1210/js.2017-00092. PMC 5686634. PMID 29264533.
- ^ Fletcher RS, Lavery GG (2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (1): R107–R121. doi: 10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- ^ James Theoga Raj, C; Lin, SJ (October 2019). "Cross-talk in NAD(+) metabolism: insights from Saccharomyces cerevisiae". Current Genetics. 65 (5): 1113–1119. doi: 10.1007/s00294-019-00972-0. PMC 6744962. PMID 30993413.
- ^ Trammell, SAJ; Brenner, C (2013). "Targeted, LCMS-based Metabolomics for Quantitative Measurement of NAD(+) Metabolites". Comput Struct Biotechnol J. 4 (5): e201301012. doi: 10.5936/csbj.201301012. PMC 3962138. PMID 24688693.
- ^ Tempel, W; Rabeh, WM; Bogan, KL; Belenky, P; Wojcik, M; Seidle, HF; Nedyalkova, L; Yang, T; Sauve, AA; Park, HW; Brenner, C (2 October 2007). "Nicotinamide riboside kinase structures reveal new pathways to NAD+". PLOS Biology. 5 (10): e263. doi: 10.1371/journal.pbio.0050263. ISSN 1545-7885. PMC 1994991. PMID 17914902.
- ^ Nam, TS; Park, DR; Rah, SY; Woo, TG; Chung, HT; Brenner, C; Kim, UH (September 2020). "Interleukin-8 drives CD38 to form NAADP from NADP(+) and NAAD in the endolysosomes to mobilize Ca(2+) and effect cell migration". FASEB Journal. 34 (9): 12565–12576. doi: 10.1096/fj.202001249R. ISSN 1530-6860. PMID 32717131.
- ^ a b c Diguet, N; Trammell, SAJ; Tannous, C; Deloux, R; Piquereau, J; Mougenot, N; Gouge, A; Gressette, M; Manoury, B; Blanc, J; Breton, M; Decaux, JF; Lavery, GG; Baczkó, I; Zoll, J; Garnier, A; Li, Z; Brenner, C; Mericskay, M (22 May 2018). "Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy". Circulation. 137 (21): 2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. ISSN 1524-4539. PMC 6954688. PMID 29217642.
- ^ a b Fons, NR; Sundaram, RK; Breuer, GA; Peng, S; McLean, RL; Kalathil, AN; Schmidt, MS; Carvalho, DM; Mackay, A; Jones, C; Carcaboso, ÁM; Nazarian, J; Berens, ME; Brenner, C; Bindra, RS (22 August 2019). "PPM1D mutations silence NAPRT gene expression and confer NAMPT inhibitor sensitivity in glioma". Nature Communications. 10 (1): 3790. Bibcode: 2019NatCo..10.3790F. doi: 10.1038/s41467-019-11732-6. ISSN 2041-1723. PMC 6706443. PMID 31439867.
- ^ a b c Vaur, P; Brugg, B; Mericskay, M; Li, Z; Schmidt, M S.; Vivien, D; Orset, C; Jacotot, E; Brenner, C (December 2017). "Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration". FASEB Journal. 31 (12): 5440–5452. doi: 10.1096/fj.201700221RR. ISSN 1530-6860. PMID 28842432.
- ^ a b Covarrubias, AJ; Kale, A; Perrone, R; Lopez-Dominguez, JA; Pisco, AO; Kasler, HG; Schmidt, MS; Heckenbach, I; Kwok, R; Wiley, CD; Wong, HS; Gibbs, E; Iyer, SS; Basisty, N; Wu, Q; Kim, IJ; Silva, E; Vitangcol, K; Shin, KO; Lee, YM; Riley, R; Ben-Sahra, I; Ott, M; Schilling, B; Scheibye-Knudsen, M; Ishihara, K; Quake, SR; Newman, J; Brenner, C; Campisi, J; Verdin, E (November 2020). "Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages". Nature Metabolism. 2 (11): 1265–1283. doi: 10.1038/s42255-020-00305-3. ISSN 2522-5812. PMC 7908681. PMID 33199924.
- ^ a b Pirinen, E; Auranen, M; Khan, NA; Brilhante, V; Urho, N; Pessia, A; Hakkarainen, A; Kuula, J; Heinonen, U; Schmidt, MS; Haimilahti, K; Piirilä, P; Lundbom, N; Taskinen, MR; Brenner, C; Velagapudi, V; Pietiläinen, KH; Suomalainen, A (2 June 2020). "Niacin Cures Systemic NAD(+) Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy". Cell Metabolism. 31 (6): 1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. hdl: 10138/330502. ISSN 1550-4131. PMID 32386566. S2CID 218585981.
- ^ Lautrup, S; Sinclair, DA; Mattson, MP; Fang, EF (1 October 2019). "NAD(+) in Brain Aging and Neurodegenerative Disorders". Cell Metabolism. 30 (4): 630–655. doi: 10.1016/j.cmet.2019.09.001. ISSN 1550-4131. PMC 6787556. PMID 31577933. S2CID 203653485. Retrieved 6 February 2023.
- ^ "Manipulating biosynthesis pathways may hold promise for preventing chemotherapy-induced pain | Carver College of Medicine". Carver College of Medicine, The University of Iowa. October 8, 2018. Retrieved 6 February 2023.
- ^ Vitali, L; Merlini, A; Galvagno, F; Proment, A; Sangiolo, D (19 October 2022). "Biological and Exploitable Crossroads for the Immune Response in Cancer and COVID-19". Biomedicines. 10 (10): 2628. doi: 10.3390/biomedicines10102628. ISSN 2227-9059. PMC 9599827. PMID 36289890.
- ^ Belenky, P; Racette, FG; Bogan, KL; McClure, JM; Smith, JS; Brenner, C (4 May 2007). "Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+". Cell. 129 (3): 473–84. doi: 10.1016/j.cell.2007.03.024. PMID 17482543. S2CID 4661723.
- ^ Trammell, SAJ; Weidemann, BJ; Chadda, A; Yorek, MS; Holmes, A; Coppey, LJ; Obrosov, A; Kardon, RH; Yorek, MA; Brenner, C (2016). "Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice". Scientific Reports. 6: 26933. Bibcode: 2016NatSR...626933T. doi: 10.1038/srep26933. PMC 4882590. PMID 27230286.
- ^ Ear, PH; Chadda, A; Gumusoglu, SB; Schmidt, MS; Vogeler, S; Malicoat, J; Kadel, J; Moore, MM; Migaud, ME; Stevens, HE; Brenner, C (22 January 2019). "Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring". Cell Reports. 26 (4): 969–983.e4. doi: 10.1016/j.celrep.2019.01.007. ISSN 2211-1247. PMID 30673618.
- ^ "Supplement makes (mouse) moms' milk better; pups benefit for life". medicalxpress.com. Retrieved 2019-01-26..
- ^ "Charles Brenner". scholar.google.com. Retrieved 3 February 2023.
- ^ Brenner, Charles; Duggan, David, eds. (2004). Oncogenomics: Molecular approaches to cancer. Hoboken, N.J.: Wiley-Liss. ISBN 0-471-22592-4.
- ^ Ritchie, Stuart (24 January 2023). "Why you shouldn't get too excited at claims scientists have reversed ageing... yet". inews.co.uk. Retrieved 3 February 2023.
- ^ Colbert, Chris (13 May 2022). "Unpacking Longevity: The Myths of Anti-Aging". Private Medical. Retrieved 2 February 2023.
- ^ Garth, Eleanor (2 November 2022). "Charles Brenner: longevity is not a simple engineering problem". Longevity.Technology - Latest News, Opinions, Analysis and Research. Retrieved 3 February 2023.
- ^ Brenner, C (12 August 2022). "Longevity lessons Methuselah's Zoo: What Nature Can Teach Us About Living Longer, Healthier Lives Steven N. Austad MIT Press, 2022. 320 pp". Science. 377 (6607): 718. doi: 10.1126/science.add9130. ISSN 0036-8075. PMID 35951694. S2CID 251516708.
- ^ Brenner, C (January 2023). "A science-based review of the world's best-selling book on aging". Archives of Gerontology and Geriatrics. 104: 104825. doi: 10.1016/j.archger.2022.104825. ISSN 0167-4943. PMC 9669175. PMID 36183524.
- ^ Brenner, C (2022-09-22). "Sirtuins are not conserved longevity genes". Life Metabolism (2): 122–133. doi: 10.1093/lifemeta/loac025. ISSN 2755-0230. PMC 10081735. PMID 37035412.
- ^ Brenner, Charles; Ringe, Dagmar (2012). "Response to the New MCAT: ASBMB Premedical Curriculum Recommendations" (PDF). ASBMB Today. No. March. pp. 12–14.
- ^ Chemical Sciences, Roundtable; Board on Chemical Sciences and Technology; Division on Earth and Life Studies; National Research Council (24 March 2014). "Chapter 1: Introduction and Overview". Undergraduate Chemistry Education: A Workshop Summary. Washington, D.C.: National Academies Press. p. 1. doi: 10.17226/18555. ISBN 978-0-309-29589-5. PMID 24830064.
- ^ a b Sengupta, Samarpita (March 1, 2016). "Brenner recognized for devotion to 'cutting-edge education' and serving the biomedical community". ASBMB Today. Retrieved 2 February 2023.
- ^ "Sirtris Pharmaceuticals, Inc. offering of common stock, Filed Pursuant to Rule 424(b)(4) Registration No.: 333-140979". www.sec.gov. May 22, 2007. Retrieved 2 February 2023.
- ^ "Charles Brenner Ph.D." Bloomberg. Retrieved 1 August 2018.
- ^ "ChromaDex Chief Scientific Advisor Dr. Charles Brenner Receives 2020 National Scientific Achievement Award from the American Society for Nutrition". BusinessWire. May 21, 2020.
- ^ "AAAS Members Elected as Fellows". American Association for the Advancement of Science (AAAS). 30 November 2012. Retrieved 2 February 2023.
- ^ "William E. M. Lands Lectureship | Biological Chemistry | Michigan Medicine". Biological Chemistry. 22 March 2018. Retrieved 2 February 2023.
- The Brenner Lab Brenner web page at City of Hope
- Brenner twitter profile
- Interviews and podcasts
- Google scholar
Charles Brenner | |
|---|---|
| Born | October 30, 1961 |
| Nationality | American |
| Alma mater |
Wesleyan University (
BA) Stanford University ( PhD) Brandeis University |
| Known for | Discovery and characterization of nicotinamide riboside as a vitamin |
| Awards | Fellow of the American Association for the Advancement of Science |
| Scientific career | |
| Fields | Enzymology Metabolism |
| Institutions |
City of Hope National Medical Center University of Iowa Dartmouth Medical School Thomas Jefferson University |
| Thesis | Specificity and Activity of the Kex2 Protease: From Yeast Genetics to Enzyme Kinetics (1993) |
| Doctoral advisor | Robert S. Fuller |
| Other academic advisors |
Gregory A. Petsko Dagmar Ringe |
| Notable students | Peter A. Belenky, Samuel A.J. Trammell |
| Website |
brennerlab |
Charles Brenner (born October 30, 1961) is the inaugural Alfred E Mann Family Foundation Chair of the Department of Diabetes & Cancer Metabolism at the Beckman Research Institute of the City of Hope National Medical Center. Brenner previously held the Roy J. Carver Chair in Biochemistry and was head of biochemistry at the University of Iowa. [1] [2]
Brenner is a major contributor in the field of nicotinamide adenine dinucleotide (NAD) metabolism and has developed targeted, quantitative methods for NAD metabolomics. [3] Brenner discovered eukaryotic nicotinamide riboside (NR) kinase and nucleosidase pathways to NAD. [4] [5] Brenner's work includes the first human trial of NR, which demonstrated safe oral availability as an NAD+ precursor. [6] [4] He has characterized ways in which NAD is disrupted by diseases and metabolic stress. [2]
Brenner graduated from Wesleyan University with a bachelor's degree in biology in 1983. After working for the biotechnology companies Chiron Corporation and DNAX Research Institute, Brenner attended graduate school at Stanford University School of Medicine. At Stanford he worked with Robert S. Fuller, receiving his Ph.D. in Cancer Biology in 1993. Brenner conducted post-doctoral research at Brandeis University with Gregory Petsko and Dagmar Ringe. [7] [8]
Brenner then joined the faculty at Thomas Jefferson University, where he worked from 1996-2003, becoming Director of the Structural Biology & Bioinformatics Program in 2000. He moved to Dartmouth Medical School in 2003, serving as Associate Director for Basic Sciences at Norris Cotton Cancer Center (now named Dartmouth Cancer Center) from 2003-2009. In 2009 he joined the University of Iowa (UI) as Professor and Departmental Executive Officer (DEO) of Biochemistry. In 2010 he became the Roy J. Carver Chair of Biochemistry at UI, holding that position until 2020. [9] [2] [10]
In 2020, Brenner joined City of Hope National Medical Center in Duarte, California as the inaugural Alfred E Mann Family Foundation Chair in Diabetes and Cancer Metabolism. City of Hope created the position and the associated Department of Diabetes & Cancer Metabolism to focus on underlying metabolism and the intersection of metabolic disturbances with diseases such as cancer and diabetes. [2] [1]
Brenner has been funded by agencies including the March of Dimes, [11] the Burroughs Wellcome Fund, [11] the Beckman Foundation, [12] the Lung Cancer Research Foundation, [13] the Bill & Melinda Gates Foundation, [14] the Leukemia & Lymphoma Society, the National Science Foundation. and the National Institutes of Health. [15]
Brenner has made multiple contributions to molecular biology and biochemistry, beginning with purification and characterization of the Kex2 proprotein convertase at Stanford. [16] [17] Significant research projects include the role of Ap3A bindings in the function of the FHIT tumor suppressor gene, [18] characterization and inhibition of DNA methylation, [19] [20] and discovery of new steps in nicotinamide adenine dinucleotide (NAD) metabolism. [21]
Notably, the Brenner laboratory discovered that eukaryotes use nicotinamide riboside (NR) to make NAD+. Bieganowski and Brenner (2004) found that NR is converted to NAD+ through the action of nicotinamide ribose kinases including Nrk1 (yeast and human) and Nrk2 (human). Belenky et al (Cell, 2007) reported another pathway which turns NR into NAM through the action of nucleosidases Urh1/Pnp1/Meu1 and is Nrk1 independent. [4] [22] [6] [23]

Brenner has developed targeted, quantitative analysis of the NAD+ metabolome [3] [24] and made fundamental contributions to NAD metabolism including discovery of nicotinic acid riboside-dependent NAD synthesis, [25] elucidating the mechanism of synthesis of nicotinic acid adenine dinucleotide phosphate, [26] and discovering multiple conditions in which NAD metabolism is dysregulated in disease. [27] [28] [29] [30] [31] [32]
Brenner is active in translating NR technologies to treat and prevent human conditions that disturb the NAD system including cancer [29] diabetic and chemotherapeutic peripheral neuropathy, [33] [34] heart failure, [28] central brain injury, [30] inflammation, [31] mitochondrial myopathy [32] pellagra, and infections [27] such as coronavirus infection [6] [35] Brenner's work included the first human trial of NR in 2016, which demonstrated safe oral availability as an NAD+ precursor. [6] [4] Though Brenner was the first to show that NR increases SIR2 activity, improves gene silencing, and can extend yeast lifespan, [6] [36] his work has not emphasized sirtuins or nonspecific anti-aging claims and instead emphasizes how NR repairs metabolic stresses that dysregulate NAD+ [28] [30] and NADPH. [6] [37]
| External videos | |
|---|---|
Examining rodents and their offspring, Brenner has showed that rodent postpartum mothers are under severe metabolic stress to their NAD system. Supplementing rodent mothers with NR increases maternal weight loss, advances juvenile development and provides long lasting neurodevelopmental advantages into adulthood. [38] [39] [14]
Brenner is an author of more than 200 peer-reviewed publications. [40] He was the senior editor of the 2004 book, Oncogenomics: Molecular Approaches to Cancer. [41]
Brenner is both cautious and critical of research that promotes claims of anti-aging and longevity. [42] [43] [44] After writing a favorable review of Steven Austad's book Methuselah's Zoo, [45] he reviewed Lifespan: Why We Age – and Why We Don't Have To by David A. Sinclair, summarizing it as "an influential source of misinformation on longevity, featuring counterfactual claims about longevity genes being conserved between yeast and humans, the existence of supposed activators of these genes, and claimed successful age reversal in mice based on partial reprogramming." [46] Brenner published a major review of sirtuins in 2022 entitled "Sirtuins are not conserved longevity genes". [47]
| External videos | |
|---|---|
In 2012, Brenner and Dagmar Ringe developed pre-medical curriculum recommendations that would be consistent with a revised Medical College Admission Test (MCAT), following a request from the President of the American Society for Biochemistry and Molecular Biology, Suzanne Pfeffer. [48] [49] The recommendations, which include development of inorganic, organic and biochemistry coursework that is more geared toward the chemistry of bioorganic functional groups, have been further refined in academic journals. Brenner's contribution to this area was recognized by the 2016 ASBMB Award for Exemplary Contributions to Education. [50]
Brenner is a former member of the Scientific Advisory Board of Sirtris Pharmaceuticals. [51] He was a co-founder of ProHeathspan prior to its acquisition by ChromaDex, and serves as member of the scientific advisory board and chief scientific advisor to ChromaDex. [7] [52]
- 2020, Mary Swartz Rose Senior Investigator Award, American Society for Nutrition [53]
- 2016, ASBMB Award for Exemplary Contributions to Education, American Society for Biochemistry and Molecular Biology [50]
- 2013, Fellow, American Association for the Advancement of Science [54]
- 2007, William E.M. Lands Lectureship, University of Michigan Medical School [55]
- 1998-2001, New Investigator in the Pharmacological Sciences, Burroughs Wellcome Fund [11]
- 1998-2000, Basil O'Connor Scholar, March of Dimes Birth Defects Foundation [11]
- 1998-2000, Beckman Young Investigators Award, Arnold and Mabel Beckman Foundation [12]
- Brenner, C (2022-09-22). "Sirtuins are not conserved longevity genes". Life Metabolism (2): 122–133. doi: 10.1093/lifemeta/loac025. ISSN 2755-0230. PMC 10081735. PMID 37035412.
- Brenner, C (January 2022). "Viral infection as an NAD+ battlefield". Nature Metabolism. 4 (1): 2–3. doi: 10.1038/s42255-021-00507-3. ISSN 2522-5812. PMC 10155260. PMID 34980922. S2CID 245654307.
- Heer, CD; Sanderson, DJ; Voth, LS; Alhammad, YMO; Schmidt, MS; Trammell, SAJ; Perlman, S; Cohen, MS; Fehr, AR; Brenner, C (2020-10-13). "Coronavirus infection and PARP expression dysregulate the NAD Metabolome: an actionable component of innate immunity". Journal of Biological Chemistry. 295 (52): 17986–17996. doi: 10.1074/jbc.RA120.015138. PMC 7834058. PMID 33051211.
- Vaur, P; Brugg, B; Mericskay, M; Li, Z; Schmidt, M S.; Vivien, D; Orset, C; Jacotot, E; Brenner, C (December 2017). "Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration". FASEB Journal. 31 (12): 5440–5452. doi: 10.1096/fj.201700221RR. ISSN 1530-6860. PMID 28842432.
- Trammell, SAJ; Schmidt, MS; Weidemann, BJ; Redpath, P; Jaksch, F; Dellinger, RW; Li, Z; Abel, ED; Migaud, ME; Brenner, C (10 October 2016). "Nicotinamide riboside is uniquely and orally bioavailable in mice and humans". Nature Communications. 7 (1): 12948. Bibcode: 2016NatCo...712948T. doi: 10.1038/ncomms12948. PMC 5062546. PMID 27721479.
- Trammell, SAJ; Weidemann, BJ; Chadda, A; Yorek, MS; Holmes, A; Coppey, LJ; Obrosov, A; Kardon, RH; Yorek, MA; Brenner, C (2016). "Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice". Scientific Reports. 6: 26933. Bibcode: 2016NatSR...626933T. doi: 10.1038/srep26933. PMC 4882590. PMID 27230286.
- Wu, B-K; Brenner, C (2014). "Suppression of TET1-Dependent DNA Demethylation Is Essential for KRAS-Mediated Transformation". Cell Reports. 9 (5): 1827–1840. doi: 10.1016/j.celrep.2014.10.063. PMC 4268240. PMID 25466250.
- Fagan, RL; Cryderman, DE; Kopelovich, L; Wallrath, LL; Brenner, C (2013). "Laccaic Acid A Is a Direct, DNA-competitive Inhibitor of DNA Methyltransferase 1". J. Biol. Chem. 288 (33): 23858–23867. doi: 10.1074/jbc.M113.480517. PMC 3745332. PMID 23839987.
- Brenner, C (2013). "Changes in Chemistry and Biochemistry Education: Creative Responses to MCAT Revisions in the Age of the Genome". Biochemistry and Molecular Biology Education. 41 (1): 1–4. doi: 10.1002/bmb.20653. PMID 23281187. S2CID 4659938.
- Brenner, C (2013). "Rethinking Premedical and Health Professional Curricula in Light of MCAT 2015" (PDF). J. Chem. Educ. 90 (7): 807–812. Bibcode: 2013JChEd..90..807B. doi: 10.1021/ed4002738. S2CID 98274150.
- Syeda, F; Fagan, RL; Wean, M; Avvakumov, GV; Walker, JR; Xue, S; Dhe-Paganon, S; Brenner, C (2011). "The Replication Focus Targeting Sequence (RFTS) Domain is a DNA-Competitive Inhibitor of Dnmt1". J. Biol. Chem. 286 (17): 15344–15351. doi: 10.1074/jbc.M110.209882. PMC 3083197. PMID 21389349.
- Bogan, KL; Brenner, C (1 August 2008). "Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD + Precursor Vitamins in Human Nutrition". Annual Review of Nutrition. 28 (1): 115–130. doi: 10.1146/annurev.nutr.28.061807.155443. ISSN 0199-9885. PMID 18429699.
- Tempel, W; Rabeh, WM; Bogan, KL; Belenky, P; Wojcik, M; Seidle, HF; Nedyalkova, L; Yang, T; Sauve, AA; Park, HW; Brenner, C (2007-10-02). "Nicotinamide riboside kinase structures reveal new pathways to NAD+". PLOS Biology. 5 (10): e263. doi: 10.1371/journal.pbio.0050263. ISSN 1545-7885. PMC 1994991. PMID 17914902.
- Robu, ME; Larson, JD; Nasevicius, A; Beiraghi, S; Brenner, C; Farber, SA; Ekker, SC (25 May 2007). "p53 Activation by Knockdown Technologies". PLOS Genetics. 3 (5): e78. doi: 10.1371/journal.pgen.0030078. ISSN 1553-7404. PMC 1877875. PMID 17530925. S2CID 9914091.
- Belenky, P; Bogan, KL; Brenner, C (January 2007). "NAD+ metabolism in health and disease". Trends in Biochemical Sciences. 32 (1): 12–19. doi: 10.1016/j.tibs.2006.11.006. PMID 17161604.
- Belenky, P; Racette, FG; Bogan, KL; McClure, JM; Smith, JS; Brenner, C (4 May 2007). "Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+". Cell. 129 (3): 473–84. doi: 10.1016/j.cell.2007.03.024. PMID 17482543. S2CID 4661723.
- Bieganowski, P; Brenner, C (2004). "Discoveries of Nicotinamide Riboside as a Nutrient and Conserved NRK Genes Establish a Preiss-Handler Independent Route to NAD+ in Fungi and Humans". Cell. 117 (4): 495–502. doi: 10.1016/S0092-8674(04)00416-7. PMID 15137942. S2CID 4642295.
- Brenner, C; Duggan, D, eds. (2004). Oncogenomics: Molecular approaches to cancer. Hoboken, N.J.: Wiley-Liss. ISBN 0-471-22592-4.
- Trapasso, F; Krakowiak, A; Cesari, R; Arkles, J; Yendamuri, S; Ishii, H; Vecchione, A; Kuroki, T; Bieganowski, P; Pace, HC; Huebner, K; Croce, CM; Brenner, C; et al. (18 February 2003). "Designed FHIT alleles establish that Fhit-induced apoptosis in cancer cells is limited by substrate binding". Proceedings of the National Academy of Sciences of the United States of America. 100 (4): 1592–7. Bibcode: 2003PNAS..100.1592T. doi: 10.1073/pnas.0437915100. PMC 149877. PMID 12574506.
- Draganescu, A; Hodawadekar, SC; Gee, KR; Brenner, C (2000). "Fhit-Nucleotide Specificity Probed with Novel Fluorescent and Fluorogenic Substrates". J. Biol. Chem. 275 (7): 4555–4560. doi: 10.1074/jbc.275.7.4555. PMC 2556043. PMID 10671479.
- Brenner, C; Fuller, RS (1992). "Structural and Enzymatic Characterization of a Purified Prohormone-Processing Enzyme: Secreted, Soluble Kex2 Protease". Proc. Natl. Acad. Sci. 89 (3): 922–926. Bibcode: 1992PNAS...89..922B. doi: 10.1073/pnas.89.3.922. PMC 48357. PMID 1736307.
- ^ a b "Leading Biochemist Charles Brenner, PhD, Joins City of Hope as Chair of First Department Focused on Diabetes and Cancer Metabolism". OncLive. MJH Life Sciences. Aug 27, 2020. Retrieved 3 February 2023.
- ^ a b c d "Brenner stepping down as chair of Department of Biochemistry | Department of Biochemistry and Molecular Biology". University of Iowa. July 8, 2020. Retrieved 2 February 2023.
- ^ a b "Awards for Regev and Gierasch; new job for Brenner". ASBMB Today. July 20, 2020.
- ^ a b c d Katsyuba, E; Romani, M; Hofer, D; Auwerx, J (January 2020). "NAD(+) homeostasis in health and disease". Nature Metabolism. 2 (1): 9–31. doi: 10.1038/s42255-019-0161-5. ISSN 2522-5812. PMID 32694684. S2CID 214277961. Retrieved 6 February 2023.
- ^ Cercillieux, A; Ciarlo, E; Canto, C (2022-08-02). "Balancing NAD+ deficits with nicotinamide riboside: therapeutic possibilities and limitations". Cellular and Molecular Life Sciences. 79 (8): 463. doi: 10.1007/s00018-022-04499-5. ISSN 1420-9071. PMC 9345839. PMID 35918544.
- ^ a b c d e f Mehmel, M; Jovanović, N; Spitz, U (31 May 2020). "Nicotinamide Riboside-The Current State of Research and Therapeutic Uses". Nutrients. 12 (6): 1616. doi: 10.3390/nu12061616. PMC 7352172. PMID 32486488.
- ^ a b "ChromaDex To Host Key Opinion Leader Webinar on the Transforming Benefits of Nicotinamide Riboside (NR)". BusinessWire. July 7, 2022. Retrieved 3 February 2023.
- ^ "Vital Signs: Investigator Insight". Dartmouth Medicine Magazine. Vol. 28, no. 4. 2004. Retrieved 2 February 2023.
- ^ "Brenner Named Head of Biochemistry at UI Carver College of Medicine". News-releases.uiowa.edu. May 5, 2009. Archived from the original on July 20, 2011. Retrieved December 9, 2010.
- ^ "CV". Brenner Lab. Retrieved 3 February 2023.
- ^ a b c d "Curriculum Vitae | Charles Brenner Laboratory". University of Iowa. Retrieved 2 February 2023.
- ^ a b "Charles Brenner". Arnold and Mabel Beckman Foundation. Archived from the original on 2 August 2018. Retrieved 1 August 2018.
- ^ "2008 - Dartmouth Medical School - Charles Brenner, PhD". Lung Cancer Research Foundation. Retrieved 6 February 2023.
- ^ a b "Humanitarian Grant Awarded for Preclinical Study on the Impact of NAD Precursor Vitamins on Milk Bioactive Production and Brain Development in Rodents". GlobeNewswire News Room. 7 October 2019. Retrieved 6 February 2023.
- ^ "@author Charles Brenner". Grantome. Retrieved 6 February 2023.
- ^ Nakayama, K (1 November 1997). "Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins". The Biochemical Journal. 327 (3): 625–35. doi: 10.1042/bj3270625. PMC 1218878. PMID 9599222. Retrieved 6 February 2023.
- ^ Sreenivas, S; Krishnaiah, SM; Govindappa, N; Basavaraju, Y; Kanojia, K; Mallikarjun, N; Natarajan, J; Chatterjee, A; Sastry, KN (January 2015). "Enhancement in production of recombinant two-chain Insulin Glargine by over-expression of Kex2 protease in Pichia pastoris" (PDF). Applied Microbiology and Biotechnology. 99 (1): 327–36. doi: 10.1007/s00253-014-6052-5. PMID 25239036. S2CID 253777642.
- ^ Herzog, D; Jansen, J; Mißun, M; Diederichs, K; Stengel, F; Marx, A (18 May 2022). "Chemical Proteomics of the Tumor Suppressor Fhit Covalently Bound to the Cofactor Ap(3)A Elucidates Its Inhibitory Action on Translation". Journal of the American Chemical Society. 144 (19): 8613–8623. doi: 10.1021/jacs.2c00815. ISSN 0002-7863. PMC 9121386. PMID 35522782. S2CID 248554359.
- ^ Syeda, F; Fagan, RL; Wean, M; Avvakumov, GV; Walker, JR; Xue, S; Dhe-Paganon S; Brenner, C (2011). "The Replication Focus Targeting Sequence (RFTS) Domain is a DNA-Competitive Inhibitor of Dnmt1". J. Biol. Chem. 286 (17): 15344–15351. doi: 10.1074/jbc.M110.209882. PMC 3083197. PMID 21389349.
- ^ Wu, B-K; Brenner, C (2014). "Suppression of TET1-Dependent DNA Demethylation Is Essential for KRAS-Mediated Transformation". Cell Reports. 9 (5): 1827–1840. doi: 10.1016/j.celrep.2014.10.063. PMC 4268240. PMID 25466250.
- ^ Elhassan, YS; Philp, AA; Lavery, GG (1 July 2017). "Targeting NAD+ in Metabolic Disease: New Insights Into an Old Molecule". Journal of the Endocrine Society. 1 (7): 816–835. doi: 10.1210/js.2017-00092. PMC 5686634. PMID 29264533.
- ^ Fletcher RS, Lavery GG (2018). "The emergence of the nicotinamide riboside kinases in the regulation of NAD+ metabolism". Journal of Molecular Endocrinology. 61 (1): R107–R121. doi: 10.1530/JME-18-0085. PMC 6145238. PMID 30307159.
- ^ James Theoga Raj, C; Lin, SJ (October 2019). "Cross-talk in NAD(+) metabolism: insights from Saccharomyces cerevisiae". Current Genetics. 65 (5): 1113–1119. doi: 10.1007/s00294-019-00972-0. PMC 6744962. PMID 30993413.
- ^ Trammell, SAJ; Brenner, C (2013). "Targeted, LCMS-based Metabolomics for Quantitative Measurement of NAD(+) Metabolites". Comput Struct Biotechnol J. 4 (5): e201301012. doi: 10.5936/csbj.201301012. PMC 3962138. PMID 24688693.
- ^ Tempel, W; Rabeh, WM; Bogan, KL; Belenky, P; Wojcik, M; Seidle, HF; Nedyalkova, L; Yang, T; Sauve, AA; Park, HW; Brenner, C (2 October 2007). "Nicotinamide riboside kinase structures reveal new pathways to NAD+". PLOS Biology. 5 (10): e263. doi: 10.1371/journal.pbio.0050263. ISSN 1545-7885. PMC 1994991. PMID 17914902.
- ^ Nam, TS; Park, DR; Rah, SY; Woo, TG; Chung, HT; Brenner, C; Kim, UH (September 2020). "Interleukin-8 drives CD38 to form NAADP from NADP(+) and NAAD in the endolysosomes to mobilize Ca(2+) and effect cell migration". FASEB Journal. 34 (9): 12565–12576. doi: 10.1096/fj.202001249R. ISSN 1530-6860. PMID 32717131.
- ^ a b c Diguet, N; Trammell, SAJ; Tannous, C; Deloux, R; Piquereau, J; Mougenot, N; Gouge, A; Gressette, M; Manoury, B; Blanc, J; Breton, M; Decaux, JF; Lavery, GG; Baczkó, I; Zoll, J; Garnier, A; Li, Z; Brenner, C; Mericskay, M (22 May 2018). "Nicotinamide Riboside Preserves Cardiac Function in a Mouse Model of Dilated Cardiomyopathy". Circulation. 137 (21): 2256–2273. doi: 10.1161/CIRCULATIONAHA.116.026099. ISSN 1524-4539. PMC 6954688. PMID 29217642.
- ^ a b Fons, NR; Sundaram, RK; Breuer, GA; Peng, S; McLean, RL; Kalathil, AN; Schmidt, MS; Carvalho, DM; Mackay, A; Jones, C; Carcaboso, ÁM; Nazarian, J; Berens, ME; Brenner, C; Bindra, RS (22 August 2019). "PPM1D mutations silence NAPRT gene expression and confer NAMPT inhibitor sensitivity in glioma". Nature Communications. 10 (1): 3790. Bibcode: 2019NatCo..10.3790F. doi: 10.1038/s41467-019-11732-6. ISSN 2041-1723. PMC 6706443. PMID 31439867.
- ^ a b c Vaur, P; Brugg, B; Mericskay, M; Li, Z; Schmidt, M S.; Vivien, D; Orset, C; Jacotot, E; Brenner, C (December 2017). "Nicotinamide riboside, a form of vitamin B3, protects against excitotoxicity-induced axonal degeneration". FASEB Journal. 31 (12): 5440–5452. doi: 10.1096/fj.201700221RR. ISSN 1530-6860. PMID 28842432.
- ^ a b Covarrubias, AJ; Kale, A; Perrone, R; Lopez-Dominguez, JA; Pisco, AO; Kasler, HG; Schmidt, MS; Heckenbach, I; Kwok, R; Wiley, CD; Wong, HS; Gibbs, E; Iyer, SS; Basisty, N; Wu, Q; Kim, IJ; Silva, E; Vitangcol, K; Shin, KO; Lee, YM; Riley, R; Ben-Sahra, I; Ott, M; Schilling, B; Scheibye-Knudsen, M; Ishihara, K; Quake, SR; Newman, J; Brenner, C; Campisi, J; Verdin, E (November 2020). "Senescent cells promote tissue NAD(+) decline during ageing via the activation of CD38(+) macrophages". Nature Metabolism. 2 (11): 1265–1283. doi: 10.1038/s42255-020-00305-3. ISSN 2522-5812. PMC 7908681. PMID 33199924.
- ^ a b Pirinen, E; Auranen, M; Khan, NA; Brilhante, V; Urho, N; Pessia, A; Hakkarainen, A; Kuula, J; Heinonen, U; Schmidt, MS; Haimilahti, K; Piirilä, P; Lundbom, N; Taskinen, MR; Brenner, C; Velagapudi, V; Pietiläinen, KH; Suomalainen, A (2 June 2020). "Niacin Cures Systemic NAD(+) Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy". Cell Metabolism. 31 (6): 1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. hdl: 10138/330502. ISSN 1550-4131. PMID 32386566. S2CID 218585981.
- ^ Lautrup, S; Sinclair, DA; Mattson, MP; Fang, EF (1 October 2019). "NAD(+) in Brain Aging and Neurodegenerative Disorders". Cell Metabolism. 30 (4): 630–655. doi: 10.1016/j.cmet.2019.09.001. ISSN 1550-4131. PMC 6787556. PMID 31577933. S2CID 203653485. Retrieved 6 February 2023.
- ^ "Manipulating biosynthesis pathways may hold promise for preventing chemotherapy-induced pain | Carver College of Medicine". Carver College of Medicine, The University of Iowa. October 8, 2018. Retrieved 6 February 2023.
- ^ Vitali, L; Merlini, A; Galvagno, F; Proment, A; Sangiolo, D (19 October 2022). "Biological and Exploitable Crossroads for the Immune Response in Cancer and COVID-19". Biomedicines. 10 (10): 2628. doi: 10.3390/biomedicines10102628. ISSN 2227-9059. PMC 9599827. PMID 36289890.
- ^ Belenky, P; Racette, FG; Bogan, KL; McClure, JM; Smith, JS; Brenner, C (4 May 2007). "Nicotinamide riboside promotes Sir2 silencing and extends lifespan via Nrk and Urh1/Pnp1/Meu1 pathways to NAD+". Cell. 129 (3): 473–84. doi: 10.1016/j.cell.2007.03.024. PMID 17482543. S2CID 4661723.
- ^ Trammell, SAJ; Weidemann, BJ; Chadda, A; Yorek, MS; Holmes, A; Coppey, LJ; Obrosov, A; Kardon, RH; Yorek, MA; Brenner, C (2016). "Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice". Scientific Reports. 6: 26933. Bibcode: 2016NatSR...626933T. doi: 10.1038/srep26933. PMC 4882590. PMID 27230286.
- ^ Ear, PH; Chadda, A; Gumusoglu, SB; Schmidt, MS; Vogeler, S; Malicoat, J; Kadel, J; Moore, MM; Migaud, ME; Stevens, HE; Brenner, C (22 January 2019). "Maternal Nicotinamide Riboside Enhances Postpartum Weight Loss, Juvenile Offspring Development, and Neurogenesis of Adult Offspring". Cell Reports. 26 (4): 969–983.e4. doi: 10.1016/j.celrep.2019.01.007. ISSN 2211-1247. PMID 30673618.
- ^ "Supplement makes (mouse) moms' milk better; pups benefit for life". medicalxpress.com. Retrieved 2019-01-26..
- ^ "Charles Brenner". scholar.google.com. Retrieved 3 February 2023.
- ^ Brenner, Charles; Duggan, David, eds. (2004). Oncogenomics: Molecular approaches to cancer. Hoboken, N.J.: Wiley-Liss. ISBN 0-471-22592-4.
- ^ Ritchie, Stuart (24 January 2023). "Why you shouldn't get too excited at claims scientists have reversed ageing... yet". inews.co.uk. Retrieved 3 February 2023.
- ^ Colbert, Chris (13 May 2022). "Unpacking Longevity: The Myths of Anti-Aging". Private Medical. Retrieved 2 February 2023.
- ^ Garth, Eleanor (2 November 2022). "Charles Brenner: longevity is not a simple engineering problem". Longevity.Technology - Latest News, Opinions, Analysis and Research. Retrieved 3 February 2023.
- ^ Brenner, C (12 August 2022). "Longevity lessons Methuselah's Zoo: What Nature Can Teach Us About Living Longer, Healthier Lives Steven N. Austad MIT Press, 2022. 320 pp". Science. 377 (6607): 718. doi: 10.1126/science.add9130. ISSN 0036-8075. PMID 35951694. S2CID 251516708.
- ^ Brenner, C (January 2023). "A science-based review of the world's best-selling book on aging". Archives of Gerontology and Geriatrics. 104: 104825. doi: 10.1016/j.archger.2022.104825. ISSN 0167-4943. PMC 9669175. PMID 36183524.
- ^ Brenner, C (2022-09-22). "Sirtuins are not conserved longevity genes". Life Metabolism (2): 122–133. doi: 10.1093/lifemeta/loac025. ISSN 2755-0230. PMC 10081735. PMID 37035412.
- ^ Brenner, Charles; Ringe, Dagmar (2012). "Response to the New MCAT: ASBMB Premedical Curriculum Recommendations" (PDF). ASBMB Today. No. March. pp. 12–14.
- ^ Chemical Sciences, Roundtable; Board on Chemical Sciences and Technology; Division on Earth and Life Studies; National Research Council (24 March 2014). "Chapter 1: Introduction and Overview". Undergraduate Chemistry Education: A Workshop Summary. Washington, D.C.: National Academies Press. p. 1. doi: 10.17226/18555. ISBN 978-0-309-29589-5. PMID 24830064.
- ^ a b Sengupta, Samarpita (March 1, 2016). "Brenner recognized for devotion to 'cutting-edge education' and serving the biomedical community". ASBMB Today. Retrieved 2 February 2023.
- ^ "Sirtris Pharmaceuticals, Inc. offering of common stock, Filed Pursuant to Rule 424(b)(4) Registration No.: 333-140979". www.sec.gov. May 22, 2007. Retrieved 2 February 2023.
- ^ "Charles Brenner Ph.D." Bloomberg. Retrieved 1 August 2018.
- ^ "ChromaDex Chief Scientific Advisor Dr. Charles Brenner Receives 2020 National Scientific Achievement Award from the American Society for Nutrition". BusinessWire. May 21, 2020.
- ^ "AAAS Members Elected as Fellows". American Association for the Advancement of Science (AAAS). 30 November 2012. Retrieved 2 February 2023.
- ^ "William E. M. Lands Lectureship | Biological Chemistry | Michigan Medicine". Biological Chemistry. 22 March 2018. Retrieved 2 February 2023.
- The Brenner Lab Brenner web page at City of Hope
- Brenner twitter profile
- Interviews and podcasts
- Google scholar