| CAMP receptor protein | |||||||
|---|---|---|---|---|---|---|---|
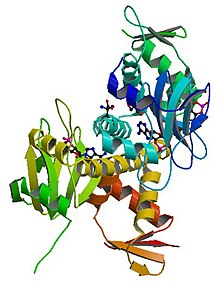 Structure of the E. coli Cyclic AMP Receptor Protein. | |||||||
| Identifiers | |||||||
| Symbol | CRP | ||||||
| Alt. symbols | CAP | ||||||
| NCBI gene | 947867 | ||||||
| PDB | 1I5Z | ||||||
| RefSeq | NP_417816.1 | ||||||
| UniProt | P0ACJ8 | ||||||
| |||||||
cAMP receptor protein (CRP; also known as catabolite activator protein, CAP) is a regulatory protein in bacteria.
Protein
CRP protein binds cyclic adenosine monophosphate (cAMP), which causes a conformational change that allows CRP to bind tightly to a specific DNA site in the promoters of the genes it controls. [1] [2] CRP then activates transcription through direct protein–protein interactions with RNA polymerase. [1] [2]
The genes regulated by CRP are mostly involved in energy metabolism, such as galactose, citrate, or the PEP group translocation system. [3] [4] In Escherichia coli, CRP can regulate the transcription of more than 100 genes.
The signal to activate CRP is the binding of cyclic AMP. Binding of cAMP to CRP leads to a long-distance signal transduction from the N-terminal cAMP-binding domain to the C-terminal domain of the protein, which is responsible for interaction with specific sequences of DNA. [5]
At "Class I" CRP-dependent promoters, CRP binds to a DNA site located upstream of core promoter elements and activates transcription through protein–protein interactions between "activating region 1" of CRP and the C-terminal domain of RNA polymerase alpha subunit. [1] [2] [6] At "Class II" CRP-dependent promoters, CRP binds to a DNA site that overlaps the promoter -35 element and activates transcription through two sets of protein–protein interactions: (1) an interaction between "activating region 1" of CRP and the C-terminal domain of RNA polymerase alpha subunit, and (2) an interaction between "activating region 2" of CRP and the N-terminal domain of RNA polymerase alpha subunit. [1] [2] At "Class III" CRP-dependent promoters, CRP functions together with one or more " co-activator" proteins. [1] [2]
At most CRP-dependent promoters, CRP activates transcription primarily or exclusively through a "recruitment" mechanism, in which protein–protein interactions between CRP and RNA polymerase assist binding of RNA polymerase to the promoter. [1]
References
- ^ a b c d e f Busby S., Ebright RH. (1999). "Transcription activation by catabolite activator protein (CAP)". J. Mol. Biol. 293 (2): 199–213. doi: 10.1006/jmbi.1999.3161. PMID 10550204.
- ^ a b c d e Lawson CL, Swigon D, Murakami KS, Darst SA, Berman HM, Ebright RH (2004). "Catabolite activator protein: DNA binding and transcription activation". Curr. Opin. Struct. Biol. 14 (1): 10–20. doi: 10.1016/j.sbi.2004.01.012. PMC 2765107. PMID 15102444.
- ^ Weickert MJ, Adhya S (1993). "The galactose regulon of Escherichia coli". Mol. Microbiol. 10 (2): 245–51. doi: 10.1111/j.1365-2958.1993.tb01950.x. PMID 7934815. S2CID 6872903.
- ^ Bott M (1997). "Anaerobic citrate metabolism and its regulation in enterobacteria". Arch. Microbiol. 167 (2–3): 78–88. doi: 10.1007/s002030050419. PMID 9133329. S2CID 22913073.
- ^ Popovych, N.; Tzeng, S. -R.; Tonelli, M.; Ebright, R. H.; Kalodimos, C. G. (2009). "Structural basis for cAMP-mediated allosteric control of the catabolite activator protein". Proceedings of the National Academy of Sciences. 106 (17): 6927–6932. doi: 10.1073/pnas.0900595106. PMC 2678429. PMID 19359484.
- ^ Hudson, B. P.; Quispe, J.; Lara-Gonzalez, S.; Kim, Y.; Berman, H. M.; Arnold, E.; Ebright, R. H.; Lawson, C. L. (2009). "Three-dimensional EM structure of an intact activator-dependent transcription initiation complex". Proceedings of the National Academy of Sciences. 106 (47): 19830–19835. doi: 10.1073/pnas.0908782106. PMC 2775702. PMID 19903881.
| CAMP receptor protein | |||||||
|---|---|---|---|---|---|---|---|
 Structure of the E. coli Cyclic AMP Receptor Protein. | |||||||
| Identifiers | |||||||
| Symbol | CRP | ||||||
| Alt. symbols | CAP | ||||||
| NCBI gene | 947867 | ||||||
| PDB | 1I5Z | ||||||
| RefSeq | NP_417816.1 | ||||||
| UniProt | P0ACJ8 | ||||||
| |||||||
cAMP receptor protein (CRP; also known as catabolite activator protein, CAP) is a regulatory protein in bacteria.
Protein
CRP protein binds cyclic adenosine monophosphate (cAMP), which causes a conformational change that allows CRP to bind tightly to a specific DNA site in the promoters of the genes it controls. [1] [2] CRP then activates transcription through direct protein–protein interactions with RNA polymerase. [1] [2]
The genes regulated by CRP are mostly involved in energy metabolism, such as galactose, citrate, or the PEP group translocation system. [3] [4] In Escherichia coli, CRP can regulate the transcription of more than 100 genes.
The signal to activate CRP is the binding of cyclic AMP. Binding of cAMP to CRP leads to a long-distance signal transduction from the N-terminal cAMP-binding domain to the C-terminal domain of the protein, which is responsible for interaction with specific sequences of DNA. [5]
At "Class I" CRP-dependent promoters, CRP binds to a DNA site located upstream of core promoter elements and activates transcription through protein–protein interactions between "activating region 1" of CRP and the C-terminal domain of RNA polymerase alpha subunit. [1] [2] [6] At "Class II" CRP-dependent promoters, CRP binds to a DNA site that overlaps the promoter -35 element and activates transcription through two sets of protein–protein interactions: (1) an interaction between "activating region 1" of CRP and the C-terminal domain of RNA polymerase alpha subunit, and (2) an interaction between "activating region 2" of CRP and the N-terminal domain of RNA polymerase alpha subunit. [1] [2] At "Class III" CRP-dependent promoters, CRP functions together with one or more " co-activator" proteins. [1] [2]
At most CRP-dependent promoters, CRP activates transcription primarily or exclusively through a "recruitment" mechanism, in which protein–protein interactions between CRP and RNA polymerase assist binding of RNA polymerase to the promoter. [1]
References
- ^ a b c d e f Busby S., Ebright RH. (1999). "Transcription activation by catabolite activator protein (CAP)". J. Mol. Biol. 293 (2): 199–213. doi: 10.1006/jmbi.1999.3161. PMID 10550204.
- ^ a b c d e Lawson CL, Swigon D, Murakami KS, Darst SA, Berman HM, Ebright RH (2004). "Catabolite activator protein: DNA binding and transcription activation". Curr. Opin. Struct. Biol. 14 (1): 10–20. doi: 10.1016/j.sbi.2004.01.012. PMC 2765107. PMID 15102444.
- ^ Weickert MJ, Adhya S (1993). "The galactose regulon of Escherichia coli". Mol. Microbiol. 10 (2): 245–51. doi: 10.1111/j.1365-2958.1993.tb01950.x. PMID 7934815. S2CID 6872903.
- ^ Bott M (1997). "Anaerobic citrate metabolism and its regulation in enterobacteria". Arch. Microbiol. 167 (2–3): 78–88. doi: 10.1007/s002030050419. PMID 9133329. S2CID 22913073.
- ^ Popovych, N.; Tzeng, S. -R.; Tonelli, M.; Ebright, R. H.; Kalodimos, C. G. (2009). "Structural basis for cAMP-mediated allosteric control of the catabolite activator protein". Proceedings of the National Academy of Sciences. 106 (17): 6927–6932. doi: 10.1073/pnas.0900595106. PMC 2678429. PMID 19359484.
- ^ Hudson, B. P.; Quispe, J.; Lara-Gonzalez, S.; Kim, Y.; Berman, H. M.; Arnold, E.; Ebright, R. H.; Lawson, C. L. (2009). "Three-dimensional EM structure of an intact activator-dependent transcription initiation complex". Proceedings of the National Academy of Sciences. 106 (47): 19830–19835. doi: 10.1073/pnas.0908782106. PMC 2775702. PMID 19903881.