| |
| Names | |
|---|---|
|
Preferred IUPAC name
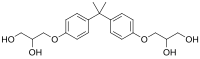
3,3′-[Propane-2,2-diylbis(4,1-phenyleneoxy)]di(propane-1,2-diol) | |
| Other names
BADGE·2H2O
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.024.524 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C21H28O6 | |
| Molar mass | 376.449 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Bis-HPPP (2,2-bis[4(2,3-hydroxypropoxy)phenyl]propane) is an organic compound that is formed when the dental composite material bis-GMA is degraded by salivary esterases. [1] It is also called BADGE·2H2O in reference to it being the hydrolyzed form of BADGE, which is used in the formation of epoxy resins. [2] Structurally, it is a di- ether of bisphenol A.
Formation
Together with methacrylic acid, bis-HPPP is released following the CE-catalyzed[ clarification needed] hydrolysis of 2,2-[4(2-hydroxy 3-methacryloxypropoxy)-phenyl]propane (bis-GMA). This reaction is very common in hydrolytic degradation of the dental resin since salivary esterases are able to cleave the ester bonds in acrylic polymers of dental composites.
Analysis by mass spectrometry demonstrated that hydrolytic reactions would cleave the ester bonds of both methacrylate units in bis-GMA and produce bis-HPPP along with two molecules of methacrylic acid. [3]
References
- ^ Shokati, Babak; Tam, Laura Eva; Santerre, J. Paul; Finer, Yoav (2010). "Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 94 (1): 230–7. doi: 10.1002/jbm.b.31645. PMID 20524199.
- ^ Yonekubo, Jun; Hayakawa, Kazuichi; Sajiki, Junko (1 March 2008). "Concentrations of Bisphenol A, Bisphenol A Diglycidyl Ether, and Their Derivatives in Canned Foods in Japanese Markets". Journal of Agricultural and Food Chemistry. 56 (6): 2041–2047. doi: 10.1021/jf073106n.
- ^ Finer Y, S.J.; Santerre, JP (2004). "The influence of resin chemistry on a dental composite's biodegradation". J Biomed Mater Res. 69A (2): 233–246. doi: 10.1002/jbm.a.30000. PMID 15057996.

| |
| Names | |
|---|---|
|
Preferred IUPAC name
3,3′-[Propane-2,2-diylbis(4,1-phenyleneoxy)]di(propane-1,2-diol) | |
| Other names
BADGE·2H2O
| |
| Identifiers | |
3D model (
JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.024.524 |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C21H28O6 | |
| Molar mass | 376.449 g·mol−1 |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Bis-HPPP (2,2-bis[4(2,3-hydroxypropoxy)phenyl]propane) is an organic compound that is formed when the dental composite material bis-GMA is degraded by salivary esterases. [1] It is also called BADGE·2H2O in reference to it being the hydrolyzed form of BADGE, which is used in the formation of epoxy resins. [2] Structurally, it is a di- ether of bisphenol A.
Formation
Together with methacrylic acid, bis-HPPP is released following the CE-catalyzed[ clarification needed] hydrolysis of 2,2-[4(2-hydroxy 3-methacryloxypropoxy)-phenyl]propane (bis-GMA). This reaction is very common in hydrolytic degradation of the dental resin since salivary esterases are able to cleave the ester bonds in acrylic polymers of dental composites.
Analysis by mass spectrometry demonstrated that hydrolytic reactions would cleave the ester bonds of both methacrylate units in bis-GMA and produce bis-HPPP along with two molecules of methacrylic acid. [3]
References
- ^ Shokati, Babak; Tam, Laura Eva; Santerre, J. Paul; Finer, Yoav (2010). "Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface". Journal of Biomedical Materials Research Part B: Applied Biomaterials. 94 (1): 230–7. doi: 10.1002/jbm.b.31645. PMID 20524199.
- ^ Yonekubo, Jun; Hayakawa, Kazuichi; Sajiki, Junko (1 March 2008). "Concentrations of Bisphenol A, Bisphenol A Diglycidyl Ether, and Their Derivatives in Canned Foods in Japanese Markets". Journal of Agricultural and Food Chemistry. 56 (6): 2041–2047. doi: 10.1021/jf073106n.
- ^ Finer Y, S.J.; Santerre, JP (2004). "The influence of resin chemistry on a dental composite's biodegradation". J Biomed Mater Res. 69A (2): 233–246. doi: 10.1002/jbm.a.30000. PMID 15057996.