| |
| |
| Names | |
|---|---|
|
Preferred IUPAC name
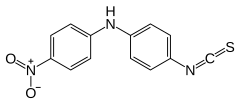
4-Isothiocyanato-N-(4-nitrophenyl)aniline | |
| Other names
4-Isothiocyanato-4′-nitrodiphenylamine
Nithiocyamine | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C13H9N3O2S | |
| Molar mass | 271.29 g·mol−1 |
| Melting point | 204 to 206 °C (399 to 403 °F; 477 to 479 K) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Amoscanate ( INN), also known as nithiocyamine, is an experimental anthelmintic agent of the aryl isothiocyanate class which was found to be highly effective in animals against the four major species of schistosomes which infect humans, [1] and is also highly active against hookworm infection. [2] [3] However, significant liver toxicity was seen in lab animals at higher doses. The ether analogue of amoscanate, nitroscanate, is used in veterinary medicine as an anthelmintic.[ citation needed]
Amoscanate was developed by Ciba. [4] [5]
- ^ Shapiro TA, Were JB, Talalay P, et al. (September 1986). "Clinical evaluation of amoscanate in healthy male volunteers". Am. J. Trop. Med. Hyg. 35 (5): 945–53. doi: 10.4269/ajtmh.1986.35.945. PMID 3766854.
- ^ Doshi JC, Vaidya AB, Sen HG, Mankodi NA, Nair CN, Grewal RS (July 1977). "Clinical trials of a new anthelmintic, 4-isothiocyanato-4'-nitrodiphenylamine (C.9333-Go/CGP 4540), for the cure of hookworm infection". Am. J. Trop. Med. Hyg. 26 (4): 636–9. doi: 10.4269/ajtmh.1977.26.636. PMID 889004.
- ^ Singh DS, Bala-Subramaniam R, Bhatia VN, Kumar V, Chandrasekar S (1981). "Study of the efficacy of compound Go.9333 (Ciba-Geigy) in hookworm infestation". Chemotherapy. 27 (3): 220–3. doi: 10.1159/000237981. PMID 7014129.
- ^ Vaidya AB, Sen HG, Mankodi NA, Paul T, Sheth UK (August 1977). "Phase 1 tolerability and searching dose studies with 4-isothiocyanato-4'-nitrodiphenylamine (C.9333-Go/CGP 4540), a new anthelmintic". Br J Clin Pharmacol. 4 (4): 463–7. doi: 10.1111/j.1365-2125.1977.tb00763.x. PMC 1429051. PMID 901739.
- ^ Sharma S (1987). "Helminth diseases". In Jucker E (ed.). Progress in Drug Research. Vol. 31. Boston: Birkhauser. ISBN 3-7643-1837-6. Retrieved on September 10, 2008 through Google Book Search.

| |

| |
| Names | |
|---|---|
|
Preferred IUPAC name
4-Isothiocyanato-N-(4-nitrophenyl)aniline | |
| Other names
4-Isothiocyanato-4′-nitrodiphenylamine
Nithiocyamine | |
| Identifiers | |
3D model (
JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem
CID
|
|
| UNII | |
CompTox Dashboard (
EPA)
|
|
| |
| |
| Properties | |
| C13H9N3O2S | |
| Molar mass | 271.29 g·mol−1 |
| Melting point | 204 to 206 °C (399 to 403 °F; 477 to 479 K) |
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
| |
Amoscanate ( INN), also known as nithiocyamine, is an experimental anthelmintic agent of the aryl isothiocyanate class which was found to be highly effective in animals against the four major species of schistosomes which infect humans, [1] and is also highly active against hookworm infection. [2] [3] However, significant liver toxicity was seen in lab animals at higher doses. The ether analogue of amoscanate, nitroscanate, is used in veterinary medicine as an anthelmintic.[ citation needed]
Amoscanate was developed by Ciba. [4] [5]
- ^ Shapiro TA, Were JB, Talalay P, et al. (September 1986). "Clinical evaluation of amoscanate in healthy male volunteers". Am. J. Trop. Med. Hyg. 35 (5): 945–53. doi: 10.4269/ajtmh.1986.35.945. PMID 3766854.
- ^ Doshi JC, Vaidya AB, Sen HG, Mankodi NA, Nair CN, Grewal RS (July 1977). "Clinical trials of a new anthelmintic, 4-isothiocyanato-4'-nitrodiphenylamine (C.9333-Go/CGP 4540), for the cure of hookworm infection". Am. J. Trop. Med. Hyg. 26 (4): 636–9. doi: 10.4269/ajtmh.1977.26.636. PMID 889004.
- ^ Singh DS, Bala-Subramaniam R, Bhatia VN, Kumar V, Chandrasekar S (1981). "Study of the efficacy of compound Go.9333 (Ciba-Geigy) in hookworm infestation". Chemotherapy. 27 (3): 220–3. doi: 10.1159/000237981. PMID 7014129.
- ^ Vaidya AB, Sen HG, Mankodi NA, Paul T, Sheth UK (August 1977). "Phase 1 tolerability and searching dose studies with 4-isothiocyanato-4'-nitrodiphenylamine (C.9333-Go/CGP 4540), a new anthelmintic". Br J Clin Pharmacol. 4 (4): 463–7. doi: 10.1111/j.1365-2125.1977.tb00763.x. PMC 1429051. PMID 901739.
- ^ Sharma S (1987). "Helminth diseases". In Jucker E (ed.). Progress in Drug Research. Vol. 31. Boston: Birkhauser. ISBN 3-7643-1837-6. Retrieved on September 10, 2008 through Google Book Search.